Diversity and genetic structure of Prochilodus magdalenae (Pisces: Prochilodontidae) upstream and downstream Betania dam, Colombia
Diversidad y estructura genética del Prochilodus magdalenae (Pisces: Prochilodontidae) aguas arriba y abajo de la represa Betania, Colombia
*Corresponding author: paupol001@gmail.com
Received: 17 April 2018 - Accepted: 11 July 2018
Abstract
The Magdalena River is one of the most important basins in Colombia, it has a large number of important fish species for the economy of many communities in the country. However, there’s been a reduction in the population caused by different factors. One of the major problems is the construction of the Betania dam, in response to the energy requirements in Colombia. However, migratory species such as Prochilodus magdalenae, of economic importance for the artisanal fishery, have been seriously affected. For this reason, it became neccesary to analyze its genetic diversity and habitat, taking into account the construction of the dam as a possible factor of fragmentation. For this purpose, the use of microsatellites as a molecular marker in different study locations upstream and downstream of the dam is adequate. It was possible to evaluate in 171 individuals the state of genetic diversity. In this respect, 111 alleles distributed in seven loci 100 % polymorphic were obtained. The overall averages for the heterozygosity observed were 0.2169, while the expected ones were 0.8316. The Fst and PhiPT statistics showed that there is a moderate differentiation between Betania’s locality and all the localities sampled. The analysis of Bayesian inference detected the coexistence of three populations in the river basin, being the Betania’s locality represented only by one of the three populations registered in the basin. These results can be presented due to the fragmentation generated by the dam in the river, preventing the genetic flow between the locations upstream and downstream of the dam.
Keywords: rheophilics fish; microsatellites; ecosystem fragmentation; genetic variability
Resumen
El río Magdalena es una de las cuencas más importantes de Colombia, cuenta con gran cantidad de especies de peces importantes para la economía de muchas comunidades en el país. Sin embargo, una reducción marcada en las poblaciones de distintas especies atribuido a diferentes factores. Dentro de estos, se destaca la construcción de la represa de Betania, como respuesta a los requerimientos energéticos en Colombia. No obstante, especies migratorias como Prochilodus magdalenae, de importancia económica para la pesquería artesanal, se han visto seriamente afectadas, por lo que se ha generado la necesidad de analizar el hábitat y la diversidad genética, teniendo en cuenta la construcción de la represa como posible factor de fragmentación. Para esto, se hace adecuado el uso de microsatélites como marcador molecular en diferentes localidades de estudio aguas arriba y abajo de la represa. Esto permitió evaluar el estado de la diversidad genética en 171 organismos, cómo se encuentra distribuida esta variabilidad en las localidades de estudio y cómo influyen algunos factores como la construcción de la represa sobre su estructura genética. En este sentido, se obtuvieron 111 alelos distribuidos en siete loci 100 % polimórficos. El promedio general para la heterocigosidad observada fue de 0,2169, mientras que para las esperadas fue de 0,8316. Los estadísticos de Fst y PhiPT mostraron que existe una moderada diferenciación entre la represa de Betania y las demás localidades muestreadas. El análisis de inferencia bayesiana detectó la coexistencia de tres poblaciones en la cuenca del río, entre las que destaca la de Betania. Estos resultados se pueden estar presentando, debido a la fragmentación que genera la represa en el río, impidiendo el flujo genético entre las localidades aguas arriba y aguas abajo de la represa.
Palabras claves: peces reofílicos; microsatélites; fragmentación de ecosistemas; variabilidad
Introduction
Fragmentation is considered one of the main factors responsible for the alteration of the environment and ecosystems; since it affects the function, structure, and composition in a given natural space (Alexander von Humboldt Institute, 2002). These effects cause a decrease in connectivity and/or create edges in the habitat, which generates changes in the behavior of species associated with fragmented environments and, therefore, increases the vulnerability of organisms (Bustamante and Grez, 1995). Despite the recognized effects of habitat fragmentation in ecosystem dynamics, the construction of artificial barriers, such as dams, is linked to the economic and social development of a community, to provide solutions to the requirement of resources such as water and energy for the populations human beings (Agostinho et al., 2008; Jiménez-Segura et al., 2014). However, its construction has come to generate from reductions or increases in the number of fish species (Agostinho et al., 2001), until local extinctions associated with the intervened watershed (Rodríguez, 2015).
It should be noted that fragmentation impedes the flow of migratory species in riverbeds (Pompeu et al., 2009). Due to the above, the decompressed construction of dams in South America has been seriously deteriorating the lotic systems, especially the biotic component (Kopas and Puentes, 2009). Thus, there have been cases in which some species of fish have increased or, if not, they have decreased due to the construction of the dam (Agostinho et al., 2008). This problem has aroused the interest of the international scientific community to understand how populations of rheophilic species respond to the construction of these physical barriers at the molecular level. This is how it is necessary to implement and use genetic tools to analyze the loss of variability in affected populations.
Therefore, the use of microsatellites has been very useful to analyze genetic aspects in global terms of many migratory fish species, such as the genus Prochilodus, a suitable biological model to understand how these organisms respond genetically to different natural and artificial barriers that can affect them (Carvalho-Costa et al., 2008; Jiménez-Segura et al., 2010; Silva, 2011; Barroca et al., 2012a; Orozco and Narváez, 2014; Pelecice et al., 2014; Guevara, 2015;).
The Magdalena River, considered as the central hydrographic basin of the country, is one of the most important means of economic support, for example, for many communities whose livelihood is based on fishing (Jiménez-Segura et al., 2010). It houses around 167 species of fish, of which Prochilodus magdalenae can be highlighted (Steindachner, 1879); endemic species, commonly known as bocachico (Maldonado-Ocampo et al., 2005), which is exploited by the artisanal fishery in the basin. This is part of the fish family Prochilodontidae and is characterized by a very sharp predorsal spine, smallmouth, prominent and small teeth, as seen in figure 1 (Mojica et al., 2012). The bocachico as a migratory species has two annual reproductive peaks that agree with the hydrological patterns of the Magdalena River. In this sense, the first reproductive peak starts between December and January, while the second, between July and September. In the latter, it is believed that only those individuals who could not do so during the first hydrological cycle of the river are reproduced (Jiménez-Segura et al., 2010).
Due to its importance in fisheries, this species has been subjected to ecological (Jiménez-Segura et al., 2010, Ramírez and Pinilla, 2012), reproductives (Atencio, 2001; Cortés, 2003) and conservation studies (Cortés, 2003; Maldonado -Ocampo et al., 2005), but there have been few works at the molecular level in Colombia. Nevertheless, among the studies carried out with microsatellites, those carried out for the species Prochilodus magdalenae (Santacruz-Beltrán, 2003, Orozco and Narváez, 2014; Guevara, 2015) can be highlighted (see table 5); where they are displayed for all cases low variability values due to different factors that include overfishing, artificial and natural barriers, among others, which allows demonstrating the critical state of the species (Orozco and Narváez, 2014).
Therefore, considering the geographic distribution of this species (Maldonado-Ocampo et al., 2005) and its category of threat as vulnerable (Mojica et al., 2012), the use of microsatellites as molecular markers is useful to evaluate the structure and genetic diversity of the bocachico after the construction of the Betania dam in 1987, in the middle and upper part of the Magdalena river basin.
Materials and methods
Study area
The Magdalena river basin occupies an area of 257438 km2, representing 24 % of the national territory, which also borders on 18 departments where about 80 % of the population depends on this basin as it is an essential axis of economic development (Lasso et al., 2011). It has three divisions that are: Alto Magdalena, with an approximate height from its birth at 3685 meters above sea level (m.s.n.m.), to Honda at 229 m.s.n.m. The second division, called Medio Magdalena, goes from 229 to 33 m.s.n.m. in the municipality of El Banco Province of Magdalena. Finalizing, the third division or under Magdalena understands the mouth of the Magdalena river that goes from 33 m.s.n.m. up to sea level (IDEAM and Cormagdalena, 2001).
Field phase
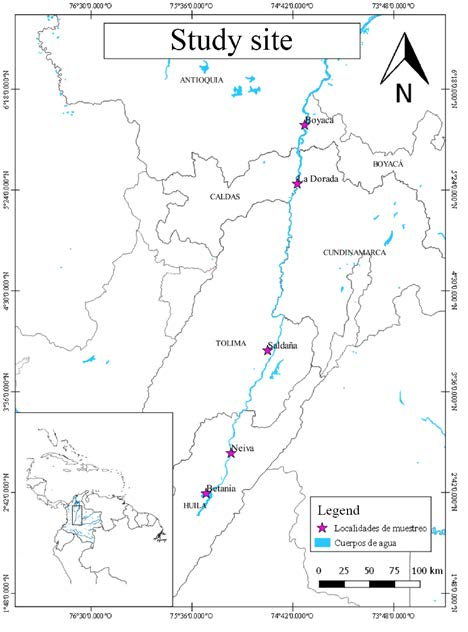
The samples were obtained with the help of the artisanal fleet and fishing landings in the five locations described in figure 2. It was also taking into account the care of the samples gathering of the muscle tissue along with the artisanal fleet since such vessels did not have much autonomy and always fish near the landing site, which guaranteed that the captured individuals belonged to the selected localities.
Because the species analyzed in this study is very commercial, its recognition in the field did not require specialized taxonomic keys. Despite, it was taken into account that indeed the individuals had the morphological characteristics such as the prickly predorsal spine, ten rays in the dorsal fin, 11 rays in the anal fin and between 40 and 46 perforated scales that characterize the lateral line (Mojica et al., 2012)
A total of 171 specimens were collected in the main channel of the river in four sites located downstream of the Betania dam on the Magdalena River and in tributaries such as the Saldaña River, as well as a site upstream of the dam, between April 2010 and November 2012 (figure 2). 40 individuals belonging to the town of Boyacá, another 40 from La Dorada, 19 from Neiva, 29 from Betania and finally 43 from Saldaña. The sample size of the present study depended on the number of specimens that were collected by fishermen in each location during the sampling period. After this, muscle samples were taken from the caudal peduncle and fixed in 96 % ethanol for preservation.
Laboratory phase
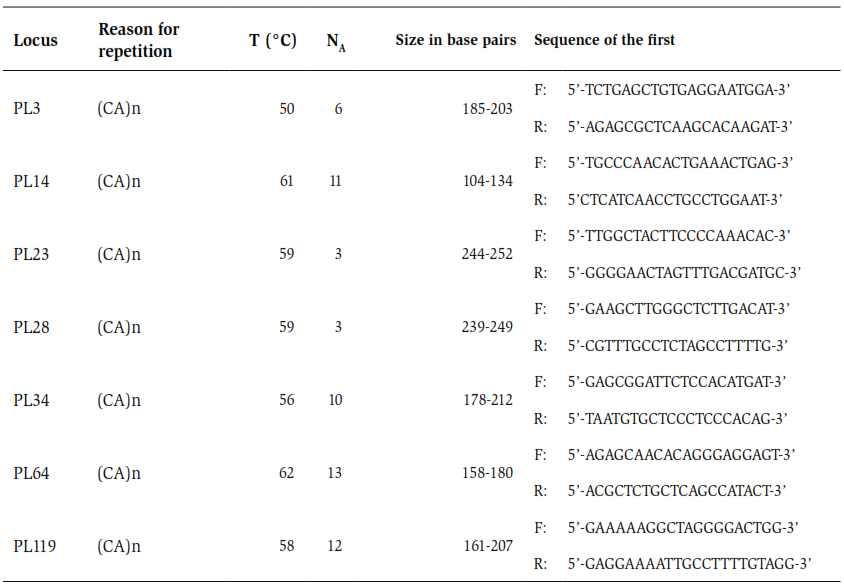
Genomic DNA was extracted from the caudal fin muscle of the collected specimens, using the MasterPurekit (Epicenter Biotechnologies®). The DNA was verified through a 0.8 % agarose gel stained with GelRed and a run at 80 volts for 30 minutes. Seven loci of microsatellite were used (table 1), described for P. lineatus with a cross-amplification in P. magdalenae (Rueda et al., 2011). The amplification reaction was carried out following the conditions proposed by Rueda et al. (2011) and Orozco and Narváez (2014). The final volume used was 10 μl, containing 100 ng of DNA, 1 X of buffer reaction, 2 mM of MgCl2, 200 μM of dNTPs, 0.2 μM of each primer and 0.25 U of Taq polymerase. The conditions for PCR were as follows: 5 minutes at 95 °C, 30 cycles of 30 s at 94 °C, 30s for the alignment temperature (table 1), 30s at 72 °C and a final extension temperature of 72 °C for 10 minutes. The reactions were carried out in a thermocycler with ESCO-SWIFT MaxPro temperature gradient. The amplification products were analyzed by QIAxcel Advanced capillary electrophoresis (QIAGEN) using a high-resolution kit (QIAGEN High-Resolution Kit) and a molecular weight ladder of known concentration (DNA SizeMarker 50-800 bp v2.0 QIAGEN). The size of each amplified product was determined with the ScreenGel program QIaxcel v1.0 QIAGEN, which allowed to quantify the weight of each band and, consequently, to distinguish the genotype of each.
Analysis of data
From the final data matrix containing the genotype of all the individuals and with the help of the MSTOOLS program, the difference between the sizes of the alleles present in the population was verified (Park, 2001). This same program was used to generate the different input files used in other programs. For example, the presence of errors in genotyping derived from technical artifacts, or loss of alleles due to poor DNA quality was evaluated with the MICRO-CHECKER program (Van Oosterhout et al., 2004), resampling the alleles of each locus to through 1000 bootstrap simulations; in this way, it creates confidence intervals between the expected frequency of homozygotes and heterozygotes for the registered alleles.
The genetic diversity was determined for each population by analyzing the number of alleles per locus (NA), observed heterozygosity (Ho) and expected heterozygosity (He), using the GenAlex 6.5 program (Peakall and Smouse, 2006). The inbreeding coefficient (Fis) for the seven loci were calculated in FSTAT (Goudet, 1995). The Hardy-Weinberg equilibrium (p < 0,05) was calculated using a test analogous to Fisher’s exact test (Guo and Thompson, 1992), which hypothesizes the random union of gametes. This test was estimated with a series of permutations of the Monte Carlo Markov Chain (MCMC) (10000 batches / 1000 iterations), implemented in GENEPOP (Raymond and Rousset, 1995).
Table 1. Description of each microsatellite used. Reason for repetition, Temperature °C, NA.; Number of alleles, Size in base pairs. Taken and modified from Rueda et al. (2011).
The independent segregation of the genotypes or linkage disequilibrium was verified using exact tests with MCMC (10000 batches / 1000 iterations) (Guo and Thompson, 1992), implemented in GENEPOP. To verify the distribution of molecular variance among natural groups of populations, the existence of genetically differentiated populations of P. magdalenae between the localities of the main channel of the Magdalena River and the dam; and to evaluate the degree of significance of the genetic variability between and within the localities, a Molecular Variance Analysis (AMOVA) was performed in the ARLEQUIN 3.1 program (Schneider et al., 2000).
This analysis examined the distribution of genetic variability within different hierarchical levels at different geographic scales in the Magdalena River basin. In this case, the hierarchical levels were evaluated as follows: 1, all the populations as a group; 2, a group composed of the localities present in the main channel in the Magdalena River without the tributaries (Saldaña River) and 3, finally considering the Betania Dam as a group independent of the other localities.
In addition to this, the ARLEQUIN 3.1 program was used to calculate the Fst values in pairs (Weir and Cockerham, 1984) estimated for each population pair. The Fst was used, since this is the index most used for the estimation of gene distances in fish populations (O’connell and Wright, 1997), besides being the most adequate in studies containing samples of moderate to small size (less than 50) and that they use less than ten loci. Additionally, the PhiPT index was used as an analogous test, based on the hypothesis that the loci are not under the assumptions of The Hardy-Weinberg equilibrium.
The Bayesian grouping methodology of STRUCTURE version 2.3.3 (Hubisz et al., 2009) was applied to identify groups of genetically similar individuals and determine the level of genetic structure in the independent data of the sampled areas. This program performs an assignment test where individuals are classified according to the probabilities of belonging to one or more groups when the population is genetically mixed.
To estimate the number of subpopulations (K), 20 independent runs of K = 1 - 8 were carried out with 10,000 MCMC repetitions, with 1000 burn-in periods, using LOC-PRIOR information and assuming correlated allele frequencies (Allele Frequencies Correlated). The pattern of migration of P. magdalenae includes the existence of strong gene flow between the populations; therefore, the model of the mixture (Admixture Model) was used, which assumes that each individual has the same proportion of inheritance of its ancestor in each population. It was used a method proposed by Evanno et al. (2005) to determine the number of populations (K) present. This value was obtained using the STRUCTURE HARVESTER 0.56.3 program (Earl and von Holdt, 2012).
Results
Genetic diversity
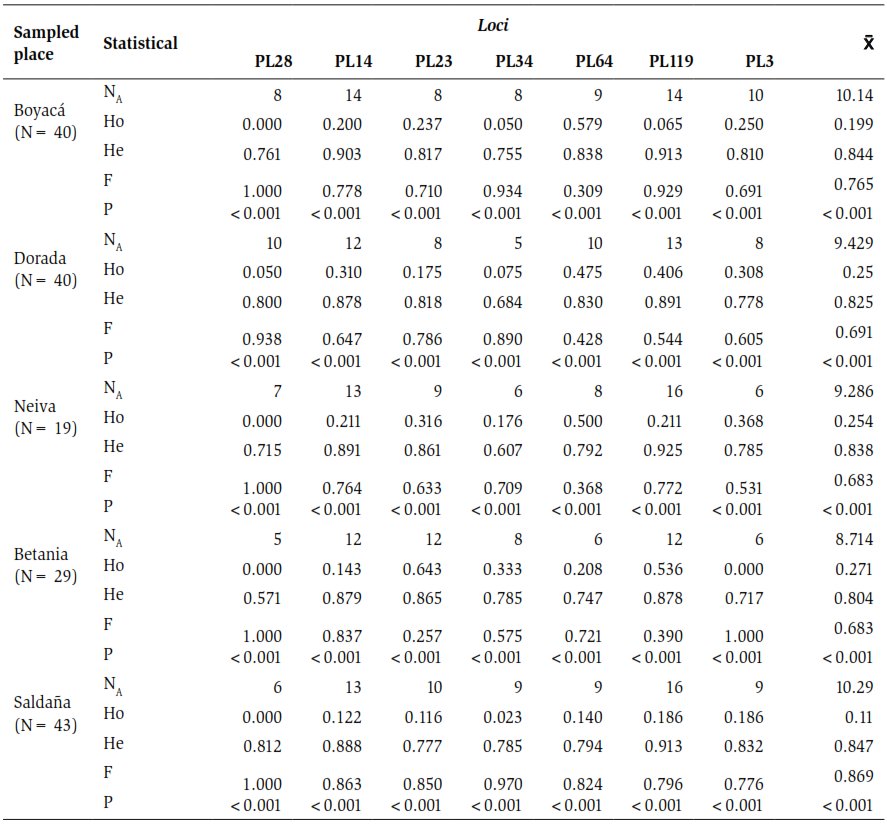
All the microsatellites evaluated were polymorphic (100 %) in the P. magdalenae specimens in the different Magdalena River localities where the samples were taken. A total of 111 alleles were found distributed in the five sampling sites. Bethany, with an average of 8.7 alleles per locus, was the least amount, while Saldana, with 10.25, was the highest average as observed in table 2. loci PL28 and PL34 had a lower amount of alleles with five each, while P119 had 16 (table 2).
On the other hand, the Ho on average for all localities was 0.21, the population of Saldaña had the lowest index with 0.11 and Bethany was the highest Ho with 0.27. However, the He was considerably higher than the Ho, with averages for all locations of 0.83. Values between 0.80 and 0.84 were obtained for the sampling sites of Betania and Saldana respectively, as shown in table 2. All the sampled localities showed a Hardy-Weinberg imbalance associated with a deficit of heterozygotes. Additionally, it was found that all microsatellite loci used in the present study had null alleles in the analyzed specimens and there was no linkage disequilibrium.
Genetic structure and population differentiation
The Fst test showed a global value of 0.065 and indicated a moderate differentiation among the localities examined, according to the scale of Wright (1978). Between each pair of populations, this index showed fluctuations ranging from locations with low differentiation such as Boyacá - La Dorada (Fst of 0.007), to locations with moderate differentiation such as La Dorada and Saldaña (Fst of 0.094), as observed in table 3 lower diagonal. Like the Fst values, the PhiPT index showed values ranging from low to moderate genetic differentiation between most localities. It is important to note that, despite the small geographic distance between the town of Neiva and the Betania dam, the values recorded for these indices showed a moderate genetic differentiation between these locations.
Table 2. General table of results. N: sample size, NA: number of alleles, Ho: observed heterozygosity, He: expected heterozygosity, F: fixation index and P: Hardy-Weinberg equilibrium.
Because all localities showed deviations in the Hardy-Weinberg equilibrium, the nonparametric test was performed analogously to Fst: PhiPT to estimate the genetic differentiation between the localities. The test showed that between the localities of Boyacá-La Dorada and Neiva-La Dorada, the lowest values were registered for this index, which indicates a low differentiation between these localities. In the other paired analyzes between localities, moderate genetic differentiation was found, as shown in table 3 above diagonal. It should be noted that the Betania dam registers the highest values for this index.
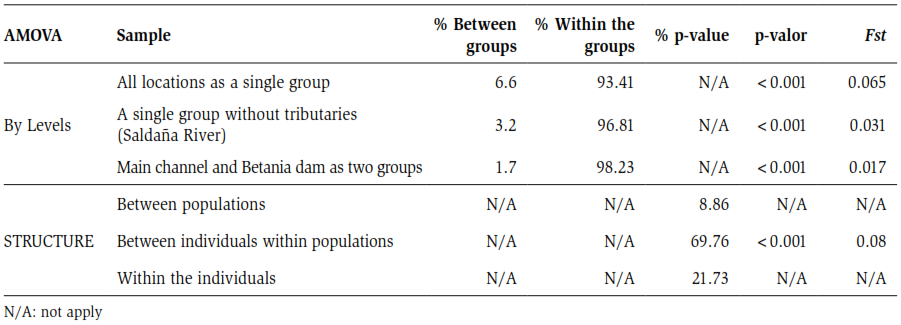
The AMOVA, based on the grouping of the localities sampled at different levels or hierarchical scales, showed that when taking all the sampled localities as a single group, an Fst value of 0.065 was recorded indicating a moderate differentiation according to the Wright scale (1978). Approximately 6.6 % of the diversity detected by the microsatellites is distributed among the populations, while about 93.41 % is distributed within the populations, as shown in table 4.
The objective of the second grouping was to evaluate the effect of tributaries of the Magdalena River on the distribution of the population’s genetic variation. In this second group, the Saldaña River was eliminated from the analysis, and an Fst value of 0.031 was found indicating the absence of genetic differentiation between the localities considered (table 4). The third group aimed to assess whether the construction of the barrier affected the population’s gene pool. The above showed a low value in the Fst index (0.017), as indicated in table 4.
However, the AMOVA and the Fst and PhiPT indices require an a priori definition of the subpopulations or localities sampled, and this grouping may not correspond to right biological groups. Therefore, to verify the veracity of the differentiation found, a Bayesian analysis was carried out in the Structure program, which identified the presence of three populations that coexist in the Magdalena river basin, as shown in figure 3.
Most of the individuals from the Boyacá and La Dorada localities were assigned to the first cluster (Green), while in the case of Neiva and Betania, the majority of the individuals were assigned to the second cluster (Red). Finally, most of the individuals present in the Saldaña River were identified as a population assigned to the third cluster (Blue).
An additional AMOVA was performed, taking into account the grouping of the individuals by cluster indistinctly from the locality of origin, to corroborate the results of the Bayesian analysis obtained in the STRUCTURE program. The results shown in table 4 indicate a moderate differentiation for the Fst statistic of 0.08 with a high level of significance.
Table 3. Lower diagonal; estimation of the Fst index among pairs of P. magdalenae localities of the five localities sampled, using seven microsatellite loci. Upper diagonal; Estimation of the PhiPT index among pairs of P. magdalenae individuals from the five sampled sites, using seven microsatellite loci.
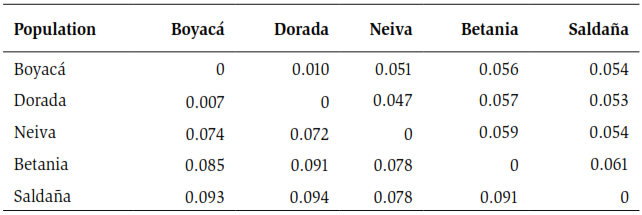
Table 4. Molecular analysis of variance (AMOVA) in the localities of P. magdalenae.
Discussion
Genetic diversity
The growing demand on the energy resource in Colombia has generated the need to strengthen the hydroelectric field with the construction of dams that affect the ichthyofauna of the Magdalena River. In Colombia, factors such as the change in the supply of food, increase in invasive species or reduction of populations with some fishing interest have been documented, due to the construction of dams (Jiménez-Segura et al., 2014). Mojica et al. (2012) show the historical evolution of the total captures of P. magdalenae in the Magdalena River, where is observed the gradual decrease that the populations of the species have suffered; coinciding also with the construction of the Betania dam in 1987. However, other more visible anthropogenic factors on the fishing resource, such as overfishing or water pollution, are not ruled out (Jiménez-Segura et al., 2014; Martínez-Silva, 2015).
These effects are not only observable at the ecosystem level (Agostinho et al., 1993); also, as in other species, at a molecular level, it has been shown that the damming of rivers influences the low genetic diversity of the species. Such is the case reported by Carvalho-Costa et al. (2008) and Rueda et al. (2011) for the species Prochilodus costatus and Prochilodus lineatus in the Sao Francisco river (Brazil), where they report diversity values below 0.5. The data recorded by these authors show that between the He and Ho indices they do not show a difference greater than 0.2 in P. costatus and P. lineatus, while the data of Orozco and Narváez (2014) and Guevara (2015) for P. magdalenae in the Magdalena basin and Catatumbo, respectively, show a difference between these indices that exceeds 0.5, coinciding, in this way, with the data obtained in the present study (table 5).
In comparative terms, there are not many studies that use microsatellite markers to evaluate the genetic characteristics of migratory fish populations in South America (Olivera et al., 2009). However, the values of genetic variability registered for P. magdalenae are not comparable to those observed for their congeners, as well as for other species of commercial importance that perform long migrations, since the levels of heterozygosity observed (Ho) for the population of bocachico is among the lowest values recorded for the whole group (table 5).
It should be noted that in the study conducted by Orozco y Narváez (2014), it is pointed out that there are locations that are distant from the Betania dam; therefore it is not clear that the low variability is solely due to the fragmentation of the ecosystem generated by this dam. Nevertheless, it is not ruled out that the localized effects of dams such as Hidrosogamoso or other smaller ones found in the Magdalena River are influencing the low variability in this population. Additionally, it is essential to identify if these populations have experienced a drastic reduction in their size, since this reduction can accelerate the loss of genetic variability and moderate fixation of deleterious alleles, as well as reduce their evolutionary potential by increasing the probability of extinction of the same (Luikart and Cornuet, 1998; Vrijenhoek, 1998).
Deviations were recorded in the HW for all loci analyzed, and presented a significant heterozygous deficit, as demonstrated by the tests performed. Additionally, this deviation can be checked due to the positive values recorded for the fixation index (F). In studies conducted with fish populations, it is common to find this type of behavior (Carvalho-Costa et al., 2008; Hatanaka et al., 2006; Sanches, 2007; Silva, 2011). These authors suggest that the possible deficiency of heterozygotes in a population can be generated by sampling errors (allele underestimation), technical problems such as the presence of null alleles, drop-out of alleles or Wahlund effect (excess of homozygotes) by overlapping populations) and selection of alleles (García De León et al., 1997; Pereira et al., 2009; Xu et al., 2001).
The Wahlund effect implies the existence of more than one population in each of the localities sampled. This could be corroborated in the bocachico populations with the help of Bayesian procedures. This result is consistent with that reported by Orozco and Narváez (2014). However, to determine specifically what is the responsible cause of the deficit of heterozygotes in a population is not an easy task, since it becomes even more complicated to identify how and what are the interactions of the populations in the natural environment. Due to the above, the deficit of heterozygotes cannot be explained only with a hypothesis, since the interaction of the different factors mentioned above may be contributing to this result (Sanches, 2007).
The results obtained may be related to the migratory behavior of P. magdalenae during the year (Atencio, 2001; Cortés, 2003; Jiménez-Segura et al., 2010; Mojica et al., 2012), because factors intrinsic to the populations can also contribute to this loss of variability. An example of this is the reduction in the size of the population due to overfishing, more susceptible during the phase of reproductive migration (Jimenez-Segura et al., 2014). This leads to the loss in the population of individuals carrying adaptive genetic variability (alleles) and decreases their evolutionary potential. Another critical factor to consider is poorly managed repopulation from stocks from fish farms that may increase homozygosity in the wild.
This fact was evidenced through Bayesian procedures at the Betania dam, where a genetic homogeneity of the population established there was shown. Likewise, the genetic relationship between the localities of Neiva and Betania is highlighted, which indicates a genetic flow between them despite the existence of the barrier. This is mainly related to the manual transport of individuals and the repopulation programs carried out in the Betania dam (Fundación Humedales, 2008). The above can be a determining factor in the low variability reported in these sectors since it is unknown the management of juveniles and bocachico breeders that are being released within the dam (Guevara, 2015).
Table 5. Comparative analysis of the genetic variability indexes obtained from microsatellite molecular markers in some migratory fish species present in South American Rivers. Where Ra: Allelic wealth, Ho: Observed variability and He: Variability expected.
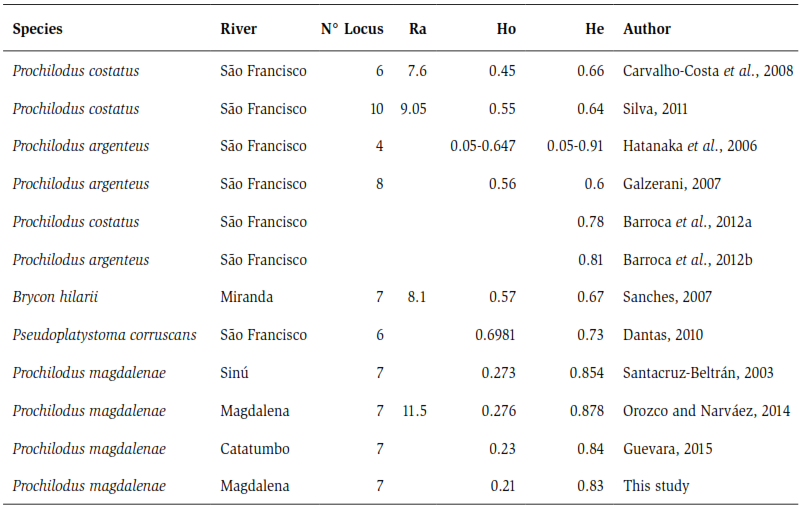
Another factor, no less important, is the fragmentation of habitat due to the filling of the swamps, which are used as feeding and breeding sites for species, construction of reservoirs and dams such as the case of Betania, which fragments the gene pool of the population. Consequently, the fragmentation and/or obstruction of migration routes alters the structure and genetic diversity of this species, and is reflected both in the values of variability obtained in this study, as well as in other biological models that present the same migratory pattern whose channels have been subject to the construction of dams (Carvalho-Costa et al., 2008; Rueda et al., 2011; Sanches et al., 2012; Orozco and Narváez, 2014; Guevara, 2015) (table 5).
The evaluated microsatellites allowed demonstrating the state of vulnerability in which the species is found, given the low genetic diversity exhibited by its population. The possibility that this low diversity is the result of the presence of null alleles and other technical artifacts and not a biological cause is not ruled out. In spite of that, it is important to highlight that these populations can be exposed to any environmental factor that can cause their local extinction (Kopas and Puentes, 2009), since upstream of the dam, the populations of endemic Magdalena species with migratory pattern of long distance, commonly known as the Dorada (Brycon moorei), the Striped Catfish (Pseudoplatystoma magdaleniatum Buitrago-Suárez and Burr, 2007) and the Picuda (Salminus affinis Steindachner, 1880) have disappeared in their entirety (Rodríguez, 2015).
Genetic structure and population differentiation
According to Laikre et al. (2005), the values of genetic variation of different populations reflect the total genetic variability of a species. This variation is vital since it allows the action of the different adaptive mechanisms in front of the challenges imposed by the environment. However, it should be noted that the distribution of genetic variation within a population is not homogeneous, and is structured in groups of genetically similar individuals (Laikre et al., 2005). This is of the utmost importance during the application of genetic methods for fisheries management since if a species has differentiated populations, management must be exercised separately (Waples et al., 2008), since it would be in front of different units of management.
The observed values for the global Fst index showed that there is a moderate genetic differentiation in the area considered (Fst = 0.065), highlighting the real differentiation between the Betania dam and all the localities considered in the analyzes. The result provided by the AMOVA indicated that there is a structure in the population of Prochilodus magdalenae at different geographical scales where this species is distributed. This differentiation can be related to several factors among which we can find, mainly, the fragmentation generated by the dam in the basin (Pelicice et al., 2014) and the effect of the tributary rivers of the Magdalena basin, as alternate sites for carrying out the reproductive event. Authors such as Carvalho-Costa et al. (2008) show, through the use of microsatellites, that there is no sub-structuring in the population of P. costatus downstream of the Tres Marías dam, located on the Sao Francisco river in Brazil. However, Barroca et al. (2012a) show that there is a marked sub-structuring in the population of this species when taking into account the individuals located upstream and downstream of the Gafanhoto dam, on the Pará river. This shows that the fish that are downstream of the dam tend to differentiate from those found upstream due to lack of gene flow. This allows us to corroborate an adverse effect of the construction of dams on migratory fish species, so more effective programs must be generated to mitigate these problems.
This is how the present study shows that the Betania dam, located on the Magdalena River, generates a population structure of P. magdalenae due to the inability of individuals to overcome this barrier and the lack of strategies to mobilize of individuals through the structure (Carvalho-Costa et al., 2008). However, despite the above, the results provided by the Bayesian analysis allowed us to observe the similarity between the Betania and Neiva dams, being part of the same population (red cluster; figure 3).
The above can be explained under two different hypotheses: (1) it is probable that this relationship is determined by reproductive events that occur upstream of the Betania dam (Jiménez-Segura et al., 2014) and eventually cross the dam evidenced by a unidirectional flow of individuals (Pelicice et al., 2014); and (2) one of the mechanisms used to reduce the impacts generated by the dams have been fish repopulation programs, where fishrelevant specimens are transported upstream of the dam (Fundación Humedales, 2008). Within these specimens is the bocachico, from fish stations whose reproducers are captured in the vicinity of the Betania dam (downstream of the dam) and released upstream in the same Carrillo-Avila et al. (2014). The inadequate management of the individuals included in the repopulation programs contributes in this way to the homogenization of the gene pool of a population (Guevara, 2015). Due to the above, in these localities, they share much genetic information that is not determined by natural migratory mechanisms.
Additionally, Orozco and Narváez (2014) and Guevara (2015) find that there is a moderate genetic differentiation for the species of P. magdalenae and P. reticulatus (synonym of P. magdalenae) respectively. This suggests the coexistence of bocachico populations in the Magdalena river basin (Orozco and Narváez, 2014). Such coexistence is maintained by the two reproductive peaks of the bocachico, which suggests that a large part of the population migrates during the first reproductive peak and in the second migrates that which did not do in the first (Jiménez-Segura et al., 2010; Orozco and Narváez, 2014). Therefore, Orozco and Narváez (2014) consider that this structuring is maintained by events of “reproductive waves,” where these genetically differentiated groups spawn in the same places at different times (Jorgensen et al., 2005). It is believed that this reproductive pattern is common in many other species within the watersheds (López-Casas et al., 2016).
It is important to highlight the genetic homogeneity existing in the Saldaña River, which is very different from the other localities. This may be evidence of the affinity that individuals may have to return to particular sites in the basin and thus carry out their reproductive process. This trend has been reported in other tropical species that make long migrations such as the bocachico (Pereira et al., 2009; Guevara, 2015). This type of behavior has also been observed in P. argenteus using tools such as radio-telemetry (Godinho and Kynard, 2006), although this is not exclusive to freshwater fish since it has also been documented in marine species (Keeney et al., 2005).
Acknowledgments
The present work could be developed within the framework of the projects: “Conservation genetics program for the Bocachico in the middle and lower watershed of the Magdalena River” (agreement number 137-09 Ecopetrol-Universidad del Magdalena) and “Evaluation of the ecology molecular of the Bocachicos (Prochilodus spp.) Associated with the rivers that drain the Colombian Caribbean “(with the code of Colciencias 1117-489-25459) of the ecology and applied biodiversity research group of the Universidad del Magdalena. Additionally, we thank all the people who contributed to the development of this work, especially María Cárdenas, Angie Angarita, and Eber Romero.
References
Agostinho, A., Vazzoler, A., Gomes, L. y Okada, E. 1993. Estratificación espacial y comportamiento de Prochilodus scrofa en distintas fases del ciclo de vida, en la planicie de inundación del alto del río Paraná y embalse de Itaipu, Paraná, Brasil. Revista de Hydrobiologia Tropical 26(1): 79-90.
Agostinho, A., Gomes, L. y Zalewski, M. 2001. The importance of floodplains for the dynamics of fish communities of the upper river Paraná. Ecohydrology and Hydrobiology 1(1): 209-217.
Agostinho, A., Pelicice, F. y Gomes, L. 2008. Dams and the fish fauna of the Neotropical region: Impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68(4): 1119-1132.
Atencio, V. 2001. Producción de alevinos de especies nativas. MVZ-Córdoba 6(1): 9-14.
Barroca, T., Arantes, F., Magalhaes, B., Siqueira, F., Horta, C., Pena, I., Dergam, J. y Kalapothakis, E. 2012a. Genetic diversity and population structure of Prochilodus costatus and Prochilodus argenteus preceding dam construction in the Paraopeba River, São Francisco River Basin, Minas Gerais, Brazil. Open Journal Genetics 2: 121-130.
Barroca, T., Santos, G., Duarte, N. y Kalapothakis, E. 2012b. Evaluation of geneic diversity population structure in a comercially important freshwater fish Prochilodus costatus (Characiformes, Prochilodontidae) using complex hypervariable repeats. Genetics and Molecular Research 1: 1 - 12.
Bustamante, R. y Grez, A. 1995. Consecuencias ecológicas de la fragmentación de Bosques Nativos. Ambiente y Desarrollo 11(2):58-63.
Carrillo-Ávila, M., Espinosa-León, L., Cruz-Flor, W. y PerdomoAguirre, Y. 2014. Caracterización genética de Ichthyoelephas longirostris de los ríos La Miel y Ranchería usando marcadores microsatélites. Orinoquía 18(2):1-10.
Carvalho-Costa, L., Hatanaka, T., y Galetti, P. 2008. Evidence of lack of population substructuring in the Brazilian freshwater fish Prochilodus costatus. Genetics and Molecular Biology 31(1): 377-380.
Cortés, G.A. 2003. Guía para el manejo, cría y conservación del Bocachico Prochilodus magdalenae Steindachner, 1879. Convenio Andrés Bello. Bogotá D. C.
Dantas, H. 2010. Avaliação da estrutura genética do surubim, Pseudoplatystoma corruscans (Actinopterigy, Siluriformes) como subsidio para o repovoamento do submédio São Francisco. Masters Thesis Universidade Federal Rural do Pernambuco, Recife, Brazil.
Earl, D. y VonHoldt, B. 2012. Structure Harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Consevation Genetics 4(2): 359-361.
Evanno, G., Regnaut, S. y Goudet, J. 2005. Detecting the number of cluster of individuals using the software Structure: A simulation study. Molecular Ecology 14: 2611-2620.
Fundación Humedales. 2008. Evaluación pesquera y repoblamientos en el embalse de Betania: Fundación Humedales. Informe final de contrato para EGEMSA S.A. Bogotá D.C.
Galzerani, F. 2007. Análise da variabilidade genética de Prochilodus argenteus (Pisces, Prochilodontidae) do rio São Francisco, região de Três Marias, através de marcadores microsatélites. Monography Genetics and Evolution, Universidade Federal de São Carlos, São Carlos, Brazil.
García De León, Chikh, F.L. y Bonhomme, F. 1997. Microsatellite polymorphism and population subdivision in natural populations of European sea bass Dicentrarchus labrax (Linneo 1758). Molecular Ecology 6:51-62.
Godinho, A. y Kynard, B. 2006. Migration and spawning of radio-tagged zulega Prochilodus argenteus in a dammed Brazilian River. Transactions of the American Fisheries Society 135: 811-824.
Goudet, J. 1995. FSTAT: A program to estimate and test gene diversities and fixation indices (version 2.9.3).
Guevara, L. 2015. Evaluación de la estructura genética de la población silvestre y cultivada del Bocachico Prochilodus reticulatus (Characiformes: Prochilodontidae) asociada a la cuenca del río Catatumbo y a centros piscícolas en el departamento del Norte de Santander. Trabajo de Grado, Universidad Francisco de Paula Santander, Ocaña.
Guo, S. y Thompson, E. 1992. Performing the exact test of Hardy-Weinberg proportion for multiple aleles. Biometrics 48: 361-372.
Hatanaka, T., Silva, F. y Galetti, P. 2006. Population substructuring in a migratory freshwater fish Prochilodus argenteus (Characiformes, Prochilodontidae) from the São Francisco River. Genetica 126:153-159.
Hubisz, M., Falush, D., Stephens, M. y Pritchard, J. 2009. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resource 9: 1322-1332.
IDEAM - Cormagdalena. 2001. Estudio ambiental de la Cuenca Magdalena-Cauca y elementos para su ordenamiento territorial. Informe técnico. Acuerdo IDEAM - Cormagdalena convenio 003 de 1999. Bogotá, D.C.
Instituto Alexander von Humboldt IAVH. 2002. Sistema de indicadores de seguimiento de la política de Biodiversidad. Unidad de Sistemas de Información Geográfica SIG. Bogotá.
Jiménez-Segura, L., Palacio, J. y Leite, R. 2010. River flooding and reproduction of migratory fish species in the Magdalena river basin, Colombia. Ecology of Freshwather Fish 19: 178-186.
Jiménez-Segura, L., Restrepo-Santamaría, D., López-Casas, S., Delgado, J., Valderrama, M., Álvarez, J. y Gómez, D. 2014. Ictiofauna y desarrollo del sector hidroeléctrico en la cuenca del río Magdalena-Cauca, Colombia. Biota Colombiana 15(2): 3 - 25.
Jorgensen, H., Hansen, M., Bekkevold, D., Ruzzante, D. y Loeschcke, V. 2005. Marine landscapes and population genetic structure of herring (Clupeaharengus L.) in the Baltic Sea. Molecular Ecology 14: 3219-3234.
Keeney, D., Heupel, M., Hueter, R. y Heist, E. 2005. Microsatellite and mitochondrial DNA analyses of the genetic structure of blacktip shark (Carcharhinus limbatus) nurseries in the northwestern Atlantic, Gulf of Mexico, and Caribbean Sea. Molecular Ecology 14: 1911-1923.
Kopas, J. y Puentes, A. 2009. Grandes represas en América ¿Peor el remedio que la enfermedad? Informe Técnico. Asociación Interamericana para la Defensa del Medio Ambiente AIDA. Oakland.
Laikre, L., Palm, S. y Ryman, N. 2005. Genetic population structure of fishes: implication s for coastal zone management. Ambio 34: 111-119.
Lasso, C., Gutierrez, F., Morales-Betancourt, M., Agudelo, E., Ramírez-Gil, H. y Ajiaco-Martínez, E. 2011. II. Pesquerías continentales de Colombia: cuencas del Magdalena-Cauca, Sinú, Canalete, Atrato, Orioco, Amazonas y vertiente del Pacífico. Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia, Instituto de Investigación de los recursos Biológicos Alexander Von Humboldt. Bogotá, D.C.
López-Casas, S., Jiménez-Segura, L., Agostinho, A. y Pérez, C. 2016. Potamodromous migrations in the Magdalena River basin: bimodal reproductive patterns in neotropical rivers. Journal of Fish Biology 1-15.
Luikart, G. y Cornuet, J. 1998. Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conservation Biology 12(1):228-237.
Maldonado-Ocampo, J., Ortega-Lara, A., Usma, J., Galvis, G., Villa-Navarro, F., Vásquez, L., Prada-Pedreros, S. y Ardila, C. 2005. Peces de los Andes de Colombia. Instituto de Investigación de Recursos BiológicosAlexander Von Humboldt. Bogotá D.C.
Martínez-Silva, P. 2015. Variación espacio-temporal de microalgas acuáticas del embalse de Betania, Huila y su relación con la calidad del agua. Intropica 10: 11-19.
Mojica, J., Usma, J., Álvarez-León, R. y Lasso, C. 2012. Libro rojo de peces dulceacuícolas de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Instituto de Ciencias Naturales de la Universidad Nacional de Colombia, WWF Colombia y Universidad de Manizales. Bogotá, D.C.
O’connell, M. y Wright, J. 1997. Microsatellite DNA in fishes. Reviews in Fish Biology and Fisheries. 7(1): 331–363.
Orozco, G. y Narváez, J. 2014. Genetic diversity and population structure of Bocachico Prochilodus magdalenae (Pisces, Prochilodontidae) in the Magdalena River basin and its tributaries, Colombia. Genetics and Molecular Biology 37 (1): 37-45.
Park, S., 2001. Trypanotolerance in Western African cattle and the population genetic effects of selection. Ph.D. Genetic Department, University of Dublin, Ireland.
Peakall, R. y Smouse, P. 2006. GenAlex6: Genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Resources (6): 288-295.
Pelicice, F., Pompeu, P. y Agostinho, A. 2014. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries 1-19.
Pereira, L., Foresti, F. y Oliveira, C. 2009. Genetic structure of the migratory catfish Pseudoplatistoma corruscans (Siluriformes: Pimelodidae) suggest homing behaviour. Ecology of Freshwater Fish 18: 215-225.
Pompeu, P., Mascarenhas, L. y Barreira, C. 2009. Evaluation of the effects of pressure gradients on four brazilianfreshwater fish species. Brazilian Archives of Biology and Technology 52 (1): 111-118.
Ramírez, A. y Pinilla, G. 2012. Hábitos alimentarios, morfometría y estados gonadales de cinco especies de peces en diferentes períodos cilmáticos en el río Sogamoso (Santander, Colombia). Acta Biológica Colombiana 17 (2): 241-258.
Raymond, M. y Rousset, F. 1995. Testing Heterozygote excess and deficiency. Genetics 140: 1413-1419.
Rodríguez, M. 2015. ¿Para dónde va el río Magdalena? Riesgos sociales, ambientales y económicos del proyecto de navegabilidad. Friedich-Ebert-Stifungen Colombia (Fescol). Bogotá D.C.
Rueda, E., Sommer, J., Scarabotti, P., Markariani, R., y Ortí, G. 2011. Isolation and characterization of polymorphic microsatellite loci in the migratory freshwater fish Prochilodus lineatus (Characiformes: Prochilodontidae). Conservation Genetics Resource 3 (4): 681-684.
Sanches, A. 2007. Estrutura genética populacional de Brycon hilarii na sub-bacia do Rio Miranda, e seu significado para programas de conservação. Doctoral Thesis, UFSCar, São Carlos, Brazil.
Sanches, A., Galetti, P., Galzerani, F., Derazo, J., Cutilak-Bianchi, B. y Hatakana, T. 2012. Genetic population structure of two migratory freshwater fish species (Brycon orthotaenia and Prochilodus argenteus) from the Sao Francisco River in Brazil and its significance for conservation. Latin American Journal of Aquatic Research 40 (1): 177-186.
Santacruz-Beltrán, D. 2003. Evaluación de la variabilidad genética conmarcadores microsatelites del Bocachico Prochilodus magdalenae (Steindachner 1878) en el Río Sinú, Colombia. Tesis de Grado, Universidad Nacional de Colombia, Bogotá.
chneider, S., Roessli, D. y Excoffier, L. 2000. Arlequin: A software for population genetics data analysis. V.2.000. Genetics and Biometry Laboratory, Dept. of Anthropology, University of Geneva, Switzerland.
Silva, A. 2011. Estrutura genética populacional de Prochilodus costatus Valenciennes 1850 (Characiformes, Prochilodontidae) no alto São Francisco. Masters Thesis, Universidade Federal de São Carlos. São Carlos, Brazil.
Van Oosterhout, C., Hutchinson, W., Wills, D. y Shipley, P. 2004. MICRO-CHECKER: Software for identifyng and correcting genotyping errors in microsatellite data. Molecular Ecology Notes 4: 535-538.
Vrijenhoek, R. 1998. Conservation genetics of freshwater fish. Journal of Fish Biology. 53: 394-412.
Waples, R., Zabel, R., Scheuerell, M. y Sanderson, B. 2008. Evolutionary responses by native species to major anthropogenic changes to their ecosystems: Pacific salmon in the Columbia River hydropower system. Molecular Ecology 17:84–96.
Weir, B., y Cockerham, C. 1984. Estimating F-statistics for the analysis of population structure. Evolution 38 (6): 1358-1370.
Wright, E. 1978. Evolution and Genetics of Population. Vol. 2: The theory of gene frequencies. Press, London: University of Chicago.
Xu, Y., Liu, Y., Ridgway, N. y McMaster, C. 2001. Novel members of the human oxysterol-binding protein family bind phospholipids and regulate vesicle transport. The Journal of Biological Chemistry 276 (21):18407-18414.
How to cite: Fontalvo P.P., Orozco-Berdugo, G. y Narváez-Barandica J.C. 2018. Diversidad y estructura genética del Prochilodus magdalenae (Pisces: Prochilodontidae) aguas arriba y abajo de la represa Betania, Colombia. Intropica 13(2): 87-100. DOI: http://dx.doi.org/10.21676/23897864.2505.