CONTRIBUCIÓN DEL 3ER CONGRESO LATINOAMERICANO DE MACROINVERTEBRADOS DE AGUA DULCE: BIODIVERSIDAD Y ECOLOGÍA FUNCIONAL EN EL NEOTRÓPICO
THE DIET OF Pomacea canaliculata (GASTROPODA: AMPULLARIIDAE) IN ITS NATIVE HABITAT BASED ON GUT CONTENT AND STABLE ISOTOPES ANALYSIS
DIETA DE Pomacea canaliculata (GASTROPODA: AMPULLARIIDAE) EN SU HÁBITAT NATURAL BASADA EN ANÁLISIS DE CONTENIDOS DIGESTIVOS E ISÓTOPOS ESTABLES
Maria Vanesa López-van Oosterom, Carolina Ocon, Ana Clara Ferreira and Alberto Rodrigues-Capítulo
Dirección de los autores:
Instituto de Limnología Dr. Raúl A. Ringuelet (CONICET-UNLP), CC 712 (1900).Tel: +54 0221 4222 775; Fax: +54 0221 4222 832, La Plata, Argentina (M.V.L.VO.), (C.O.), (A.C.F.), (A.R.C.). Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Argentina (C.O.), (A.C.F.), (A.R.C.).Consejo Nacional de Investigaciones Científicas y Técnicas (CCT-La Plata-CONICET), Argentina (M.V.L.VO.), (C.O.), (A.R.C.). Corresponding author: oosteromvan@gmail.com (M.V.L.VO.)
DOI: http://dx.doi.org/10.21676/23897864.1864
ABSTRACT
Pomacea canaliculata is native to Rio de la Plata basin, and it is invasive in most of South and Southeast Asia after accidental introduction during unsuccessful attempts to establish commercial aquaculture of this species, and being present, the number one pest of rice crops in the region. Investigations in its native habitat are still needed because fundamental issues of its trophic ecology remain unknown. The aim of this research was to study the diet of P. canaliculata in its natural habitat through gut content techniques and stable isotopes of 13C and 15N. Biological samples were taken from November 2009 to December 2010 in Carnaval stream, a characteristic Pampean stream. Samples for stable isotopes of 13C and 15N using of a mixing model were collected in November 2011. The trophic strategy diagram evinced a generalist diet with high consumption of detritus followed by the vascular plants and algae. The mixing model results indicated that the relative contribution to the diet was similar for all basal resources: fine and coarse particulate organic matter, epipelon and aquatic macrophytes (approximately 40 %). These results evidenced a strong dietary plasticity for the species.
KEY WORDS: Apple snail, feeding strategy, temperate Pampean stream.
RESUMEN
Pomacea canaliculata es una especie nativa de la Cuenca del Río de la Plata, actualmente considerada invasora en el Sur y Este de Asia donde fue introducida con fines comerciales y se ha convertido en la principal plaga de los cultivos de arroz de la región. Realizar más investigaciones en el hábitat natural de este organismo es necesario debido a que características fundamentales de su ecología trófica permanecen aún desconocidas. El objetivo de este trabajo fue estudiar la dieta de P. canaliculata en su hábitat natural mediante los análisis de contenidos digestivos e isótopos estables de 13C y 15N. Las muestras biológicas fueron tomadas desde noviembre de 2009 hasta diciembre de 2010 en el arroyo Carnaval, un arroyo pampeano característico. Las muestras para análisis de isótopos estables y posterior aplicación de modelos de mezcla fueron colectadas en noviembre de 2011. La aplicación de un diagrama de estrategia trófica permitió establecer una dieta generalista con elevado consumo de detritos, seguidos por plantas vasculares y algas. Los resultados del modelo de mezcla, sin embargo, indicaron que la contribución relativa a la dieta de todos los recursos basales evaluados (materia orgánica particulada fina y gruesa, epipelon y macrófitas acuáticas) fue similar (aproximadamente 40 %). Estos resultados evidenciaron una fuerte plasticidad dietaria para la especie.
PALABRAS CLAVE: Caracol manzana, estrategia alimentaria, arroyos templados Pampeanos.
INTRODUCTION
Pomacea canaliculata (Lamarck, 1822) (Gastropoda: Ampullariidae) is a neotropical freshwater snail native to Rio de la Plata basin (Hayes et al., 2012). It is widely distributed in ponds, marshes and slow speed streams and rivers throughout Bolivia, Paraguay, Uruguay, Brazil and Argentina (Martín et al., 2001; Burlakova et al., 2010) being a problematic specie in other countries.
Biological invasions may alter the properties of the invaded habitat, decline biodiversity and induce biotic homogenization (Alonso and Castro-Diez, 2008). Once the invader population has settled, it will compete with indigenous species for food and space (Morrison and Hay, 2011). At the beginnings of the 1980, P. canaliculata was introduced in South and Southeast Asia becoming a serious pest of rice crops causing incalculable economic losses (Carlsson et al., 2004; Morrinson and Hay, 2011; Kwong et al., 2009; Joshi and Sebastian, 2013). The favorable weather conditions, the availability of food resources (crops or macrophytes), a high reproductive potential and the lack of natural predators have contributed to its success as an invader (Damborenea and Darrigran, 2002; Joshi, 2007; Hayes et al., 2008). In consequence, this species is actually distributed in several countries of Asia and Oceania, North America (Hawaii and other states of the United States) (Rawlings et al., 2007; Hayes et al., 2008; Tran et al., 2008; Cowie and Hayes, 2012) and it was even recently reported in some regions of Spain (EFSA PLH Panel, 2013), Pakistan (Baloch et al., 2012) and Russia (Yanygina et al., 2010). The impacts of P. canaliculata are not restricted to agricultural economics, but they are also a serious concern for public health as they are a known carrier of the Rat Lung Worm (Angiostrongylus cantonensis), the causative agent of eosinophilic meningoencephalitis (Lv et al., 2009; Yang et al., 2013). Additionally, their voracious appetite impact on the ecological integrity of invaded ecosystems and threatens the survivor of native populations (Joshi, 2007; Fang et al., 2010; Hayes et al., 2015).
The plasticity in feeding is thought to be a major contributing factor to apple snail invasive success and impact as crop pest (Tamburi and Martín, 2009; Morrison and Hay, 2011). Three different kinds of feeding strategies have been described for the ampullariids: 1- microphytophagous, which is the most common, 2-zoophagous, that can include recently dead prey (“necrophagous”), macerates (“scavenger”) or even predation (Karraker and Dudgeon, 2014) and 3- microphagous, performed by scraping hard surfaces (“browsing”) or by the generation of ciliary currents (“ciliary feeding”). The latter is a particular way of feeding observed mainly in aquaria, in which the snail forms a funnel with its foot near the water surface and a ciliary current guides the small particles of food to the mouth opening (Saveanu and Martín, 2013).
Feeding preferences and mechanisms have been studied on cultured organisms of P. canaliculata (Tamburi and Martín, 2009; Wong et al., 2010; Saveanu and Martín, 2013). Also, field studies dealing with the feeding habits of this snail have been carried out in areas where this species is an invader (Qiu and Kwong, 2009; Kwong et al., 2010; Morrison and Hay, 2011). Some studies referring to different topics of the ecology of these organisms have been carried out in environments of our country more recently (Seuffert and Martín, 2009; Seuffert et al., 2010; Seuffert and Martín, 2013) but local investigations are still needed because fundamental issues of its trophic ecology in natural habitat remain unknown.
A method traditionally employed for studying the diets of invertebrates is the analysis of gut contents since it usually represents the type of food available in the environment (Allan and Castillo, 2007). However, this method presents some disaventages such as the impossibility to determine certain food categories when digestion process is advanced or consider a food item as prey when it was accidentally acquired during the feeding act. Therefore, the use of stable isotopes constitutes a key tool to discern trophic ecological process. The analysis of the tissues using this technique allows to consider only the assimilated food sources. Moreover, unlike gut content analysis, it integrates functional responses over time. The stable-N isotopic signature (δ15N) in particular, is used to determine the trophic level position of a single member or group of organisms in a given food web because the δ15N of a consumer becomes enriched relative to its prey (Vander-Zanden and Rasmussen, 2001). Stable-carbon isotopes can the indicate the feeding and carbon-flow pathways because of the occurrence of little fractionation through the food web and since different energy sources can have differing δ13C values (DeNiro and Epstein, 1981; Minagawa and Wada, 1984; Peterson and Fry, 1987; Vander-Zanden and Rasmussen, 2001). The models (e.g., MixSIR and SIAR) that have recently emerged based on stable isotope data combined with Bayesian analysis techniques (Moore and Semmens, 2008; Parnell et al., 2010; Solomon et al., 2011) incorporating many sources of variability within the model, can be adequate tools for quantifying such sources of uncertainty and for estimating the contribution of multiple sources to floodplain lake food webs. The aim of this study was, therefore, to study the diet of P. canaliculata in its natural habitat through the examination of gut contents and analysis of stable isotopes of 13C and 15N.
MATERIALS AND METHODS
Study area
Snail samples were collected from the Carnaval stream, a lotic system (stream order 1) located in Buenos Aires Province, Argentina (S 34° 53´ 8.67”; W 58° 5´ 23.43”). This stream is approximately 14.5 km long, and the sampling site is located in the middle reach. The primary land use in the upper and middle section of the basin is agriculture (flower and fruit cultures), although wastelands are also nearby. However, the lower section of the stream is surrounded by urban settlements with some industrial activity (Banda-Noriega and Ruiz de Galarreta, 2002).The main characteristics of the studied reach of the stream are: high turbidity (about 100 NTU), Total Suspended Solids (74 mg.l-1), low water flow (0.03 m3.sec-1) and a mean depth of 0.19 m. The granulometric composition of the sampling site sediment is mainly sandy-silty. Submerged and emergent vegetation composed of Sagittaria montevidensis, Alternanthera filoxeroides, Eleocharis montana, Polygonum punctatum, Hydrocotyle bonariensis, and Ludwigia peploides is found on the margins.
Sampling
A 100 m long segment was delimited in the stream and georeferenced with a Garmin III plus GPS (the same stretch was considered for the all sampling dates). In lowland streams, the sediment and the vegetation are the primary habitats for apple snails. Therefore, samples of those two habitat types (sediment and hydrophytes) were performed seasonally (November 2009 till December 2010) and collected in triplicate at the stream in order to obtain P. canaliculata densities. This was performed in order to know the representativeness of the species in the selected sites. Samples of sediment were taken with an Ekman grab (100 cm2). Samples of hydrophytes were collected within the area subsumed by a Plexiglas square of 625 cm2, while sieves were used to collect snails present in this habitat. In the laboratory, the snails were identified and dissected for gut content analyses and isotope analyses.
Gut content analysis
A total of 30 individuals was analysed for diet. The shell length (SL) was measured in all the collected organisms from the apex to the lower margin of the peristome (SL, mm) with a digital calliper. The mid-gut of individuals was then dissected for analysis. Stomach contents were placed in vials and stained with Bengal´s rose colorant for 24 h. These samples were then transferred to distilled water and mixed; fifteen fields per slide were randomly selected and food items were counted under a microscope at a 400 X magnification (Winterbourn et al., 1984; Díaz-Villanueva and Albariño, 1999). For each item, the length and area covered were measured with a graduate eyepiece, and the relative abundance was expressed as a percentage of the area occupied by the total gut content (Winterbourn et al., 1984; Jaarsma et al., 1998; Díaz-Villanueva and Albariño, 1999; Muñoz et al., 2009).
The Costello method modified by Amundsen et al. (1996) was used to determine the alimentary strategy (generalist and specialist). The diagram obtained with this method shows the feeding strategy of the consumer by the graphic representation of the relative abundance of the prey i (Pi) in relation to its frequency of occurrence (Fi).
The percentage of abundance of the prey (Pi) is calculated as follows:
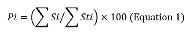
Where Σsi is the occurrence of the prey “i” in all the stomachs and ΣSti is the total content of all the stomachs where the prey “i” was found.
Frequency of occurrence (Foi) was calculated as follows:
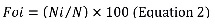
Where Ni is the number of predators with the prey “i” present in their stomachs and N is the total number of predators with stomach content.
The vertical axis represents the feeding strategy of the predator in terms of specialization or generalization. The predators have specialized on prey types positioned in the upper part of the graph, whereas prey positioned in the lower part have been eating more occasionally (generalization). Prey points located at the upper left of the diagram would be indicative of specialization of individual predators, and those in the upper right would represent specialization of the predator population. Observations located to the upper right of the diagram (population specialization) must necessarily be restricted to a single or a few points, reflecting a predator population with a narrow niche width. If there are no prey points in the upper right of the diagram, and all prey points are located along or below the diagonal from the upper left to the lower right, the predator population will have a broad niche width. Prey points are positioned in either the upper left or the lower (Amundsen et al., 1996).
Stable isotopes analysis (SIA)
We collected snails and different energy sources in five opportunities November 2009, March 2010, June 2010, September 2010, and December 2010. The basal resources recollected were fine particulate organic matter (FPOM<65 µm), coarse particulate organic matter (CPOM>65 µm), epipelic biofilm and selected macrophytes for SIA. Snails were collected on vegetation patches, taken to the laboratory and placed in different containers with filtered stream water. Depending on the body mass of snails, two or three individuals (same location and sampling date) were pooled to provide 3-4 mg samples for replicated analysis. Individuals were then fasted for 24 h to ensure the evacuation and stomach emptying, in order to include only assimilated substances in the analysis. Snail feces were siphoned out and individuals kept in a freezer at -20 °C until their processing, and the shells were mechanically removed and discarded for the isotopic and stoichiometric analyses of C and N (Bosley and Wainright, 1999).
The dominant and more frequent emergent macrophytes present through all the sampling dates (Ludwigia peploides and Hydrocotyle bonariensis) were collected by triplicate for SIA. They were sonicated for three 2-minute cycles in a Cleanson ultrasonic to remove attached algae of the plant surfaces. For epipelon analysis in each sampling date, a number of ten subsamples were collected by pipetting (Stevenson, 1984; Lowe and Laliberte, 1996) a superficial layer of 5 – 10 mm of the sediment in different places, following Descy and Coste (1990) and Gómez and Licursi (2001) recommendations.
All the material was dried to constant weight at 60 °C and then ground into a powder to insure homogeneity and analyzed in a mass spectrometer (IRMS FinniganTM MAT Delta S) coupled to an elemental analyzer (CATNAS, Montevideo, Uruguay). Isotopic ratios are typically expressed as the ratio of the high to low isotope and converted into delta notation (δ-values) through comparison of sample isotope ratios to ratios of internationally accepted standards. Standards for common systems include Vienna-Pee Dee Belemnite limestone (V-PDB) for carbon, atmospheric N2 for nitrogen. Such notation is expressed as follows:
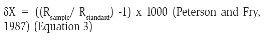
The contribution of each alimentary resource to the diet of P. canaliculata was studied through the package Stable Isotope Analysis in R (SIAR), applying the Mixing Bayesian model with the incorporation of the elemental concentration of C and N. Due to the lack of information for this region, the Trophic Enrichment Factor (TEF) used was 1.3 (0.3+/-) ‰ for δ13C and 2.2 (0.3+/-) ‰ for δ15N (McCutchan et al., 2003) considerate from a diet of plant and algal.
RESULTS
In table 1 was summarized the density (in different habitat and sampling dates), alimentary items consumed, and SL of all snails sampled. The density values sowed that in November 2009, an average density of 27 ind. m-2 (in sediment); was recorded in sediment samples while in March 2010 a density of 10 ind.m-2 of P. canaliculata was recorded in sediments and 42 ind.m-2 among stands of vegetation (A. filoxeroides). No individuals were found in the sediment samples of both June and September 2010, although some snails were collected in the sampling conducted for the gut content study. In December 2010, the density recorded was 63 ind. m-2 among stands of H. bonariensis. The average size of the analyzed individuals was 26.8 mm (SD ± 9.59 mm). Total SL (mm) of dissected snails for the study of gut content was 5.6 (n = 1) in November 2009; 36.8 ± 7.6 (n = 10) in March 2010; 18.6 ± 10.8 (n = 3) in June 2010; 21.9 ± 3.9 (n = 6) and 26.9 ± 4.1 (n = 10) in December 2010, variability in the number and length of individuals depended on abundance in the environment and in each sampling date several individuals with empty gut content were discarded from the diet analyses.
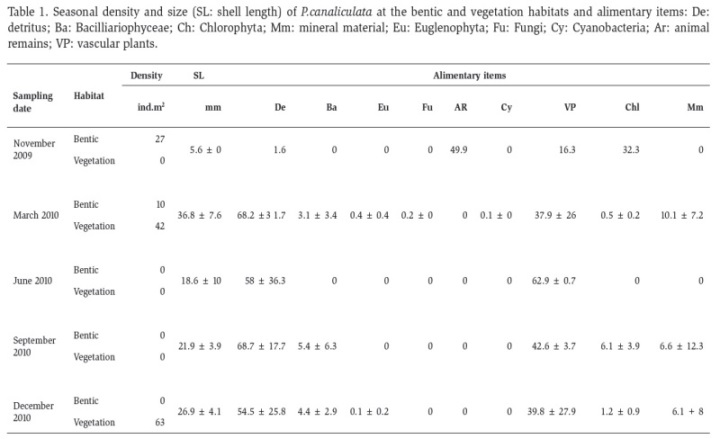
The maximum lengths of the consumed alimentary items were recorded for the vegetal remains in March, June and December 2010. The detritus achieved the maximum value of length in September 2010 and the animal remains in November 2009 (Figure 1).
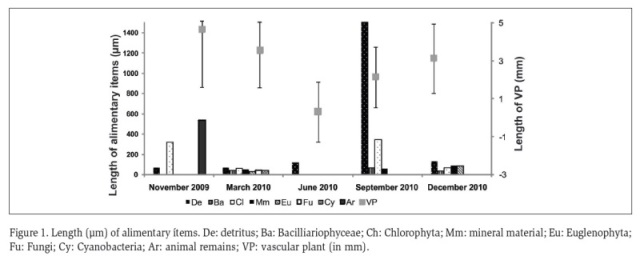
According to the Amundsen trophic strategy diagram (Figure 2), P. canaliculata has a generalist diet with high consumption of detritus (70 % of relative abundance and 100 % of occurrence frequency), followed by the vascular plants (35 % of relative abundance and 70 % of occurrence frequency). The consumption of biofilm was variable: Cyanobacteria, Fungi and Euglenophyta was low (relative abundance <4 %). Chlorophyta and Bacillariophyceae algae were consumed more frequently (% occurrence >50 %), but in low amounts (relative abundance <5 %), and animal remains were recorded infrequently (<5 % occurrence frequency) with a 10 % of relative abundance.
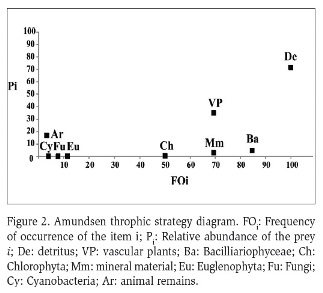
The figure 3 shows the stable nitrogen and carbon isotopes in P. canaliculata and in the basal resources available. The snails isotopic signatures averaged were for nitrogen to Stable isotopes values for snails averaged were -25.38 (± 4.63) for δ13C and 10.32 (± 2.65) for δ15N. The values of δ13C for FPMO and CPOM were of -23.9 (± 0.8) and -24.2 (± 2.4) respectively, while for the macrophytes were -29.1 (± 0.3) and 10.6 (± 0.5) for L. peploides and H. bonariensis respectively. Epipelon value of δ13C was -25.8 (± 0.8). The nitrogen values had a wide range of variation, the values for FPMO and CPOM were of 3.2 (± 2.9) and 2.9 (± 3.6) respectively. Nitrogen isotopic values (δ15N) for macrophytes were high, of 10.45 (± 0.5) for L. peploides, and 10.45 (± 3.3) for H. bonariensis. Finally, epipelon value of δ15N was of 7.3 (± 0.3).
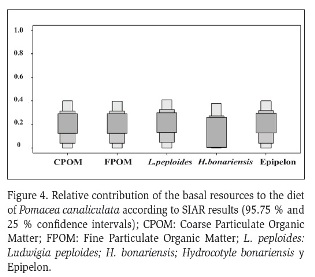
According to the SIAR results (with 95 % confidence intervals), the relative contribution of each basal resource was about 40 % for each alimentary item: FPMO and CPOM contributed with a 40 % each, H. bonariensis 39 %, L. peploides 41 % and the epipelon 40 % (Figure 4).
DISCUSSION
In accordance with Cazzaniga and Estebenet (1984) and Dillon (2000), our results show that P. canaliculata is a generalist species that feeds mainly on vascular plants (macrophytophagous), detritus and periphyton. Animal remains constituted only a small proportion of remains found in the stomach contents analyzed (e.g. Acarii, Ostracoda and Chironomidae). This pattern agrees with other authors about animal remains being included in the diet of these snails (Cazzaniga and Estebenet, 1984; Aditya and Raut, 2001, Wood et al., 2006; Kwong et al., 2009). This is in accordance with Horgan et al. (2014) who addressed that under conditions where macrophytes, algae and decaying plant materials are sufficiently abundant, predation of aquatic fauna is probably low. The distribution pattern of prey points of P. canaliculata along the diagonal in Amundsen trophic strategy diagrams indicates a high within-phenotype component in niche width, in which most of the individuals utilize many resource types simultaneously.
In the stream sampled, eight different alimentary items were found in the diet of P. canaliculata, with dominance of detritus and vascular plant remains, whereas Cyanobacteria, algae and fungi were found in low frequency and abundance. These results are comparable with those reported in wetlands of Hong Kong, where this exotic snail exhibits a secondary production greater than any of the native species. Besides, although detritus and macrophytes constitute its main diet, benthic algae are also very common in the gut and can be an additional food source for this omnivorous snail (Kwong et al., 2010).
It has been suggested that its preference for vascular plants is due to its high nitrogen content and low phenolic concentration in tissues (Wong et al., 2010; Qiu et al., 2011). Moreover, Qiu and Kwong (2009) concluded that such nutritional benefits and low chemical defenses, also found in cultivated macrophytes (Amaranthus gangeticus, Apium graveolens, Ipomea acuatica, Nasturtium officinale), could explain the successful invasion of this snail in fields cultivated with those plant species in Asia.
Mineral materials were also recorded in the stomach contents of P. canaliculata, but it is unknown whether they were part of the grit present in the gizzard that is used for triturating food (Andrews, 1965), or they were incorporated by scraping during the ingestion, taking into account that the stream studied presents a high content of fine sediment in the stream bottom (Ocon et al., 2013; López-van Oosterom et al., 2013).
The wide values of stable isotopes measured in P. canaliculata in our study were similar to those obtained for Pomacea lineata in Brazil after being fed with different C3 and C4 macrophytes (Fellerhoff, 2002), what suggests that isotope values can exhibit a wide range depending on the food source. In fact, the wide range of δ13C signatures suggests that P. canaliculata shows an heterogeneous diet composed mainly by CPOM, FPOM, epipelon and macropytes.
In an study carried out in a wetland of China, it was found that P. canaliculata was positioned in the first trophic level with other herbivorous mollusks, with a value of 5 0/00 of δ15N (Chen et al., 2016). In our study, those values were usually higher, (13 0/00 ) coinciding with results reported for different macroinvertebrates species (including P. canaliculata) in studies done in streams and large rivers of the Neotropical region (Marchese et al., 2014; Ocon et al., 2013). This could be explained by the enrichment in δ15N registered in Pampean streams due to the process of denitrification in the sediments (Acuña et al., 2010). The denitrification process due to bacterial activity increases the fractioning of nitrogen, therefore increasing the δ15N throughout the food webs (Michener and Lajtha, 2007).
The elevated nitrogen values recoded in the present study are consistent with those registered in other mollusk species such as Melanoides tricarinata, detritivore snail that, along with biofilm, consumes dead individuals or fecal pellets from other cohabiting organisms (Coat et al., 2009). Therefore, although the elevated consumption of macrophytes of P. canaliculata is already known (Cazzaniga and Estebenet, 1984; Kwong et al., 2010), the nutrient enrichment (C and N) and high amounts of detritus and biofilm found in the gut content of this snail in our study suggests that these items may also constitute an important food resource and a source of carbon for this species, such as it has been demonstrated for other aquatic organisms of the Pampean sreams (Rodrigues-Capítulo et al., 2002; Licursi and Gómez, 2003; Gómez et al., 2009).
The fact that the contribution of each feeding resource analyzed was similar and not higher than 40 % suggests that the species feed on those items indiscriminately, supporting their generalist feeding behavior as proposed in earlier studies (Cazzaniga and Estebenet, 1984; Cowie, 2002; Estebenet and Martín, 2002; Kwong et al., 2010).
Our results also indicate that P. canaliculata shows a strong dietary plasticity, being able to feed on the vegetation as well as on biofilms. In addition to this, the detritus constitutes a predominant unlimited food resource for the organisms. This agrees with the observations on the diet, where the vegetal remains represented 70 % of frequency of occurrence and detritus 100 %. This fact could indicate that when the aquatic vegetation decreases P. canaliculata can complement its diet with detritus. Being able to feed from different resources gives this snail an advantage not only in its natural environment but also in the environments where it is an invader. P. canaliculata has an important status in the energetic balance of sediments of pampean streams due to a high consumption of vascular plants and detritus (Cazzaniga and Estebenet, 1984), since the waste it produces is later used by other secondary consumers. The excretion and/or egestion of nutrients by grazers also increase the supply of nutrients available to the periphyton (Hillebrand et al., 2008).
Snails may spatially recycle nutrients, increasing the availability and uptake of nutrients by the periphyton (Mulholland et al., 1991).
In conclusion, our results confirm the polyphagous habits and dietary plasticity of P. canaliculata evidenced by the snail gut content and isotopes stables was observed many categories of alimentary items but low contribution percentage of each one in SIA. Nevertheless, future research should include other basal resources and experimental studies measuring the effects of different types and qualities of alimentation on the snail dietary preferences using resources within the natural habitat since the most effective means of controlling apple snails in an invaded environment is to understand the trophic ecology of their native environment. The combined use of a classical technique such as the analysis of stomach contents along with other more recently developed tools such as the stable-isotope ratios increases the precision of the results and assists in confirming conclusions. In this regard, future studies could also include detailed chemical analyses of P. canaliculata’s preferential diet in native habitat.
ACKNOWLEDGEMENTS
This study was supported by the project PIP 0341 CONICET. This is Scientific Contribution N° 947 from the Instituto de Limnología “Dr. Raúl A. Ringuelet” (CONICET La Plata-UNLP). We wish to thank Mónica Caviglia for editing the final version of the manuscript. We are grateful to Joaquin Cochero and Hernan Benitez for their review of the manuscript and for field assistance, respectively.
REFERENCES
Acuña, V., Vilches, C. and Giorgi, A. 2010. As productive and slow as a stream can be-the metabolism of a Pampean stream. Journal of the North American Benthological Society 30: 71–83.
Allan, J.D. and Castillo, M.M. 2007. Stream ecology: structure and function of running waters. Springer Science and Business Media, Netherlands.
Alonso, A. and Castro-Diez, P. 2008. What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)?. Hydrobiologia 614:107–116.
Amundsen, P.A., Gabler, H.M. and Staldvik, F.J. 1996. A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. Journal of fish biology 48: 607–614.
Aditya, G. and Raut, S.K. 2001. Food of the snail, Pomacea bridgesii, introduced in India. Current Science 80: 919–921.
Andrews, E.B. 1965. The functional anatomy of the gut of the prosobranch gastropod Pomacea canaliculata and of some other pilids. Proceedings of the Zoological Society of London 145(1): 19–36.
Baloch, W.A., Memon, U.N., Burdi, G.H., Soomro, A.N., Tunio, G.R. and Khatian, A.A. 2012. Invasion of channeled apple snail Pomacea canaliculata, Lamarck (Gastropoda: Ampullariidae) in Haleji Lake, Pakistan. Sindh University Research Journal (Science Series) 44(2): 263–266.
Banda-Noriega, R. and Ruiz de Galarreta, A. 2002. Riesgo de contaminación hídrica subterránea por la actividad industrial, cuenca de los arroyos Martín y Carnaval, Buenos Aires, Argentina. In: Bocanegra, E., Martínez, D. and Massone, H., Editors. Groundwater and human development. Proceedings XXXII IAH and VI ALH-SUD Congress. Mar del Plata, Argentina.
Bosley, K.L. and Wainright, S.C. 1999. Effects of preservatives and acidification on the stable isotope ratios (15N: 14N, 13C: 12C) of two species of marine animals. Canadian Journal of Fisheries and Aquatic Sciences 56: 2181–2185.
Burlakova, L.E., Padilla, D.K., Karatayev, A.Y., Hollas, D.N., Cartwright, L.D. and Nichol, K.D. 2010. Differences in population dynamics and potential impacts of a freshwater invader driven by temporal habitat stability. Biological Invasions 12: 927–941.
Cazzaniga, N.J. and Estebenet, A.L. 1984. Revisión y notas sobre los hábitos alimentarios de los Ampullariidae (Gastropoda). Historia Natural 4: 213–224.
Carlsson, N., Kestrup, Å., Mårtensson, M. and Nyström, P. 2004. Lethal and non‐lethal effects of multiple indigenous predators on the invasive golden apple snail (Pomacea canaliculata). Freshwater Biology 49: 1269–1279.
Chen, Q., Liu, Y., Ho, W.T., Chan, S.K., Li, Q.H. and Huang, J.R. 2016. Use of stable isotopes to understand food webs in Macao wetlands. Wetlands Ecology and Management 1–8 doi:10.1007/s11273-016-9502-2.
Coat, S., Monti, D., Bouchon, C. and Lepoint, G. 2009. Trophic relationships in a tropical stream food web assessed by stable isotope analysis. Freshwater Biology 54: 1028–1041.
Cowie, R.H. 2002. Apple snails (Ampullariidae) as agricultural pests: their biology, impacts, and management. In: Baker, G.M., Editor. Molluscs as crop pests. CABI Publishing, Wallingford, UK.
Cowie, R.H. and Hayes, K.A. 2012. Apple snails. In: Francis, R.A, Editor. A handbook of global freshwater invasive species. Earthscan, London.
Damborenea, C. and Darrigran, G. 2002. Un sudamericano invade Asia. Ciencia hoy 11: 24–30.
DeNiro, M.J. and Epstein, S. 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et cosmochimica acta 45(3): 341–351.
Descy, J.P. and Coste, M. 1990. Utilisation des diatomées benthiques pour l’évaluation de la qualité des eaux courantes. Rapport final, UNECED, Namur, Cemagref, Bordeaux.
Díaz-Villanueva, D. and Albariño, R.J. 1999. Feeding habit of Notoperla archiplatae (Plecoptera) larvae in a North Patagonia Andean stream, Argentina. Hydrobiologia 4: 43–52.
Dillon, R.T.Jr. 2000. Gastropod autecology. In: Dillon, R.T.Jr., Editor. The ecology of freshwater molluscs. Cambridge University Press, Cambridge.
Estebenet, A.L. and Martín, P.R. 2002. Pomacea canaliculata (Gastropoda: Ampullariidae): life-history traits and their plasticity. Biocell: official journal of the Sociedades Latinoamericanas de Microscopia Electronica 26: 83–89.
EFSA PLH Panel (EFSA Panel on Plant Health). 2013. Scientific Opinion on the assessment of the potential establishment of the apple snail in the EU. EFSA Journal 11(12): 3487 doi:10.2903/j.efsa.2013.3487.
Fang, L., Wong, P.K., Lin, L., Lan, C. and Qiu, J.W. 2010. Impact of invasive apple snails in Hong Kong on wetland macrophytes, nutrients, phytoplankton and filamentous algae. Freshwater Biology 55: 1191–1204.
Fellerhoff, C. 2002. Feeding and growth of apple snail Pomacea lineata in the Pantanal wetland, Brazil. A stable isotope approach. Isotopes in Environmental and Health Studies 38: 227–243.
Gómez, N. and Licursi, M. 2001. The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquatic Ecology 35: 173–181.
Gómez, N., Sierra, M.V., Cochero, J., Licursi, M. and Bauer, D.E. 2009. Epipelic biofilms as indicators of environmental changes in lowland fluvial systems. In: Bailey, W.C., Editor. Biofilms: Formation, Development and Properties. Nova Science Publishers, New York.
Hayes, K.A., Joshi, R.C., Thiengo, S.C. and Cowie, R.H. 2008. Out of South America: multiple origins of non‐native apple snails in Asia. Diversity and distributions 14: 701–712.
Hayes, K.A., Cowie, R.H., Thiengo, S.C. and Strong, E.E. 2012. Comparing apples with apples: clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zoological Journal of the Linnean Society 166: 723–753.
Hayes, K. A., Burks, R.L., Castro-Vazquez, A., Darby, P.C., Heras, H., Martín, P.R., Qiu, J.W., Thiengo, S.C., Vega, I.A., Wada, T., Yusa, Y., Burela, S., Cadierno, M.P., Cueto, J.P., Dellagnola, F.A., Dreon, M.S., Frassa, M.V., GiraudBilloud, M., Godoy, M.S., Ituarte, S., Koch, E., Matsukura, K., Pasquevich, M.Y, Rodriguez, C., Saveanu, L., Seuffert, M.E., Strong, E.E., Sun, J., Tamburi, N.E., Tiecher, M.J., Turner, R.L., Valentine-Darby, P.L. and Cowie, R.H. 2015. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 58(1–2): 245–302.
Hillebrand, H., Frost, P. and Liess, A. 2008. Ecological stoichiometry of indirect grazer effects on periphyton nutrient content. Oecologia 155: 619–630.
Horgan, F.G., Stuart, A.M. and Kudavidanage, E.P. 2014. Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecologica 54: 90–100.
Jaarsma, N.G., De Boer, S.M., Townsend, C.R., Thompson, R.M. and Edwards, E.D. 1998. Characterising food‐webs in two New Zealand streams. New Zealand Journal of Marine and Freshwater Research 32: 271–286.
Joshi, R.C. 2007. Problems with the management of the golden apple snail Pomacea canaliculata: an important exotic pest of rice in Asia. In: Vreysen, M.J.B., Robinson, A.S. and Hendrichs, J., Editors. Area-wide control of insect pests. Springer, Netherlands.
Joshi, R.C. and Sebastian, L.S. 2013. Golden Apple Snails. Philippine Rice Research Institute, Philippine.
Karraker, N.E. and Dudgeon, D. 2014. Invasive apple snails (Pomacea canaliculata) are predators of amphibians in South China. Biological invasions 16: 1785–1789.
Kwong, K.L., Chan, R.K. and Qiu, J. W. 2009. The potential of the invasive snail Pomacea canaliculata as a predator of various life-stages of five species of freshwater snails. Malacologia 51: 343–356.
Kwong, K.L., Dudgeon, D., Wong, P.K. and Qiu, J.W. 2010. Secondary production and diet of an invasive snail in freshwater wetlands: implications for resource utilization and competition. Biological Invasions 12: 1153–1164.
Licursi, M. and Gómez, N. 2003. Aplicación de índices bióticos en la evaluación de la calidad del agua en sistemas lóticos de la Llanura Pampeana Argentina a partir del empleo de diatomeas. Biología acuática 21: 31–49.
López-van Oosterom, M.V., Ocón, C.S., Brancolini, F., Maroñas, M.E., Sendra, E.D. and Rodrigues-Capítulo, A. 2013. Trophic relationships between macroinvertebrates and fish in a pampean lowland stream (Argentina). Iheringia. Série Zoologia 103: 57–65.
Lowe, R. and Laliberte, G.D. 1996. Benthic stream algae: distribution and structure. In: Hauer, R. and Lamberti, G.A., Editors. Stream Ecology. Academic Press, San Diego, California.
Lv, S., Zhang, Y., Liu, H.X., Zhang, C.W., Steinmann, P., Zhou, X.N. and Utzinger, J. 2009. Angiostrongylus cantonensis: morphological and behavioral investigation within the freshwater snail Pomacea canaliculata. Parasitology research 104: 1351–1359.
Marchese, M.R., Saigo, M., Zilli, F.L., Capello, S., Devercelli, M., Montalto, L. and Wantzen, K.M. 2014. Food webs of the Paraná River floodplain: Assessing basal sources using stable carbon and nitrogen isotopes. Limnologica-Ecology and Management of Inland Waters 46: 22–30.
Martín, P.R., Estebenet, A.L. and Cazzaniga, N.J. 2001. Factors affecting the distribution of Pomacea canaliculata (Gastropoda: Ampullariidae) along its southernmost natural limit. Malacologia 43: 13–23.
McCutchan, J.H., Lewis, W.M., Kendall, C. and McGrath, C.C. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378–390.
Michener, R. and Lajtha, G.W. 2007. Stable Isotopes. Blackwell publishing, Australia.
Minagawa, M. and Wada, E. 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et cosmochimica acta 48: 1135–1140.
Moore, J.W. and Semmens, B.X. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11: 470–480.
Morrison, W.E. and Hay, M.E. 2011. Feeding and growth of native, invasive and non-invasive alien apple snails (Ampullariidae) in the United States: invasives eat more and grow more. Biological Invasions 13: 945–955.
Mulholland, P.J., Steinman, A.D., Palumbo, A.V., Elwood, J.W. and Kirschtel, D.B. 1991. Role of nutrient cycling and herbivory in regulating periphyton communities in laboratory streams. Ecology 72: 966–982.
Muñoz, I., Romaní, A.M., Rodrigues-Capítulo, A., Esteban, J.G. and Berthou, E.G. 2009. Relaciones tróficas en el ecosistema fluvial. In: Elosegi, A. and Sabater, S., Editors. Conceptos y técnicas en ecología fluvial. Fundación BBVA, Bilbao, España.
Ocon, C., López-van Oosterom, M.V., Muñoz, M.I. and Rodrigues-Capítulo, A. 2013. Macroinvertebrate trophic responses to nutrient addition in a temperate stream in South America. Fundamental and Applied Limnology/ Archiv für Hydrobiologie 182: 17–30.
Parnell, A.C., Inger, R., Bearhop, S. and Jackson, A.L. 2010. Source partitioning using stable isotopes: coping with too much variation. PloS one 5: e9672. doi:10.1371/journal. pone.0009672.
Peterson, B.J. and Fry, B. 1987. Stable isotopes in ecosystem studies. Annual review of ecology and systematics 18: 293-320.
Qiu, J.W. and Kwong, K.L. 2009. Effects of macrophytes on feeding and life‐history traits of the invasive apple snail Pomacea canaliculata. Freshwater Biology 54: 1720–1730.
Qiu, J.W., Chan, M.T., Kwong, K.L. and Sun, J. 2011. Consumption, survival and growth in the invasive freshwater snail Pomacea canaliculata: does food freshness matter?. Journal of Molluscan Studies 77: 189–195.
Rawlings, T.A., Hayes, K.A., Cowie, R.H. and Collins, T.M. 2007. The identity, distribution, and impacts of nonnative apple snails in the continental United States. BMC Evolutionary Biology 7: 97. doi:10.1186/1471-2148-7–97.
Rodrigues-Capítulo, A., Paggi, A.C. and Ocón, C.S. 2002. Zoobenthic communities in relation with the slope, substrate heterogeneity and urban disturbs on pampean hills streams (Argentine). Verhandlungen des Internationalen Verein Limnologie 28: 1267–1273.
Saveanu, L. and Martín, P.R. 2013. Pedal surface collecting as an alternative feeding mechanism of the invasive apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae). Journal of Molluscan Studies 79: 11–18.
Seuffert, M.E. and Martín, P.R. 2009. Influence of Temperature, Size and Sex on Aerial Respiration of Pomacea canaliculata (Gastropoda: Ampullariidae) from Southern Pampas, Argentina. Malacologia 51:191–200.
Seuffert, M.E., Burela, S. and Martín, P.R. 2010. Influence of water temperature on the activity of the freshwater snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) at its southernmost limit (Southern Pampas, Argentina). Journal of Thermal Biology 35: 77–84.
Seuffert, M.E. and Martín P.R. 2013. Distribution of the apple snail Pomacea canaliculata in Pampean streams (Argentina) at different spatial scales. Limnologica - Ecology and Management of Inland Waters 43: 91–99.
Solomon, C.T., Carpenter, S.R., Clayton, M.K., Cole, J.J., Coloso, J.J., Pace, M.L. and Weidel, B.C. 2011. Terrestrial, benthic, and pelagic resource use in lakes: results from a three‐isotope Bayesian mixing model. Ecology 92: 1115–1125.
Stevenson, R.J. 1984. Epilithic and epipelic diatoms in the Sandusky River, with emphasis on species diversity and water pollution. Hydrobiologia 114: 161–175.
Tamburi, N.E. and Martín, P.R. 2009. Feeding rates and food conversion efficiencies in the apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae). Malacologia 51: 221–232.
Tran, C., Hayes, K.A. and Cowie, R.H. 2008. Lack of mitochondrial DNA diversity in invasive apple snails (Ampullariidae) in Hawaii. Malacologia 50(1/2): 351–357.
Vander-Zanden, M. and Rasmussen, J.B. 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and oceanography 46(8): 2061–2066.
Winterbourn, M.J., Cowie, B. and Rounick, J.S. 1984. Food resources and ingestion patterns of insects along a west coast, South Island, river system. New Zealand journal of marine and freshwater research 18: 379–388.
Wong, P.K., Liang, Y.A.N., Liu, N.Y. and Qiu, J.W. 2010. Palatability of macrophytes to the invasive freshwater snail Pomacea canaliculata: differential effects of multiple plant traits. Freshwater Biology 55: 2023–2031.
Wood, T.S., Anurakpongsatorn, P., Chaichana, R., Mahujchariyawong, J. and Satapanajaru, T. 2006. Heavy predation on freshwater bryozoans by the golden apple snail, Pomacea canaliculata Lamarck, 1822 (Ampullariidae). The Natural History Journal of Chulalongkorn University 6: 31–36.
Yang, T.B., Wu, Z.D. and Lun, Z.R. 2013. The apple snail Pomacea canaliculata, a novel vector of the rat lungworm, Angiostrongylus cantonensis: its introduction, spread, and control in China. Hawai’i Journal of Medicine and Public Health 72: 23–25.
Yanygina, L.V., Kirillov, V.V. and Zarubina, E.Y. 2010. Invasive species in the biocenosis of the cooling reservoir of Belovskaya power plant (Southwest Siberia). Russian Journal of Biological Invasions 1: 50–54.
Fecha de Recibido: 10/10/2016
Fecha de Aceptación: 21/11/2016
Para citar este artículo: López-van Oosterom, M.V., Ocon, C., Ferreira, A.C. and Rodrigues-Capítulo, A. 2016. The diet of Pomacea canaliculata (Gastropoda: Ampullariidae) in its native habitat based on gut content and stable isotopes analysis. Revista Intropica Vol. 11: 73 - 83