CONTRIBUCIÓN DEL 3ER CONGRESO LATINOAMERICANO DE MACROINVERTEBRADOS DE AGUA DULCE: BIODIVERSIDAD Y ECOLOGÍA FUNCIONAL EN EL NEOTRÓPICO
OCCURRENCE OF NEMATODES ON EPHEMEROPTERA NYMPHS IN A TROPICAL RAINFOREST STREAM
OCURRENCIA DE NEMÁTODOS EN NINFAS DE EPHEMEROPTERA EN UNA QUEBRADA DE BOSQUE TROPICAL LLUVIOSO
Limarie J. Reyes-Torres, Yazminne Meléndez-Torres and Alonso Ramírez
Dirección de los autores:
Department of Environmental Sciences, University of Puerto Rico, San Juan, Puerto Rico 00931. Tel. 787-503-9608; e-mail: limarie.reyes1@upr.edu (L.J.R.T.). Department of Biology, University of Puerto Rico, Ponce, Puerto Rico 00716. Tel. 939-325-4868; e-mail: yazminne.melendez@upr.edu (Y.M.T.). Department of Environmental Sciences, University of Puerto Rico, San Juan, Puerto Rico 00931. Tel. 787-764-0000, ext. 88194, e-mail: aramirez@ramirezlab.net (A.R.)
DOI: http://dx.doi.org/10.21676/23897864.1863
ABSTRACT
Nematodes are common symbionts of aquatic insects. Here, we assessed the presence of nematodes in mayfly nymphs (Ephemeroptera), evaluated their prevalence in the population, and determined factors associated with nematode presence. Mayflies were collected (n = 130) from three stream habitats (riffles, pools, and boulders) using a D net, in Prieta stream, El Verde Field Station, Puerto Rico. Mayflies were dissected and nematode presence was determined under a light microscope (4 x and 10 x). Nematode prevalence was 50 %. Nematodes were not identified beyond Phylum level, but we were able to eliminate various groups as infective agents (Nematoda: Mermithidae and Nematomorpha: Gordiida). They were mostly found in the abdomen, head or thorax of mayflies. There were differences in infection among taxa, Neohagenulus was the group with the highest proportion of infection. Mayfly body shape, feeding strategy, or activity potentially explain differences in infection among taxa. There were no differences in infection among habitats, but mayflies were less abundant in riffles; and there was no relation between mayfly body length and the number of nematodes present. To our knowledge, this is the first report of a nematode present in mayfly nymphs in Puerto Rico.
KEY WORDS: Mayflies, Nematoda, Nymphs, Tropical streams, Puerto Rico.
RESUMEN
Los nematodos son simbiontes comunes en los insectos acuáticos. Aquí evaluamos la presencia de nematodos en ninfas de Ephemeroptera, determinamos su prevalencia en la población y los factores asociados a la presencia del nematodo. Se colectaron ninfas (n = 130) de Ephemeroptera de tres microhábitats de río (rápidos, pozas, y rocas) usando una red D, en la quebrada Prieta, Estación de Campo El Verde, Puerto Rico. Se disectaron las ninfas y la presencia del nematodo fue determinada bajo microscopio de luz (4 x y 10 x). La prevalencia del nematodo fue de 50 %. Los nematodos presentes no fueron identificados más allá del nivel de Filo, pero se rechazaron varios grupos (Nematoda: Mermithidae y Nematomorpha: Gordiida). Los nematodos fueron más comunes en el abdomen, cabeza y tórax de los efemerópteros. Hubo diferencias en infección entre taxa, Neohagenulus fue el grupo con la mayor proporción de infección. La forma del cuerpo, estrategias de alimentación, o actividad de las ninfas, pueden potencialmente explicar las diferencias en infección entre taxa. No hubo diferencias de infección entre hábitats, pero los efemerópteros fueron menos abundantes en los rápidos; tampoco se observó una relación entre la longitud de los efemerópteros y la cantidad de nematodos presentes. Según nuestra información, este es el primer reporte de un nematodo en ninfas efemerópteras en Puerto Rico.
PALABRAS CLAVE: Efemerópteros, Nematoda, Ninfas, Quebradas tropicales, Puerto Rico.
INTRODUCTION
Nematodes are common symbionts of aquatic insects and can be found living inside or outside the organism (sensu Poinar, 1975). They are relatively common, but we have limited information on their frequency of occurrence and their biology (Poinar, 2015). Some groups are phoretic and their relation with aquatic insects is only as means of transportation or substrate (Poinar, 1975; Svensson, 1979; Sudhaus, 2008; Giblin-Davis et al., 2013). Other groups are obligate parasites and use insects as intermediate or final hosts (Poinar, 1975), as is the case of hairworms (Nematomorpha: Gordiida) (Hominick and Welch, 1980). Parasitic nematodes can have important effects on the insect population dynamics. For example, mermithid nematodes (Nematoda: Mermithidae) castrate their insect host and alter their behavior, thus functionally eliminating that individual from the population (Hominick and Welch, 1980; Vance and Peckarsky, 1996; 1997; Williams et al., 2001).
Mayflies (Ephemeroptera) are dominant insects in stream ecosystems. They play important roles in streams and are also infected by symbiotic nematodes (Hominick and Welch, 1980). Studies on temperate regions have found that nematodes are common in mayflies, in particular those of the mermithid group (Vance and Peckarsky, 1996). Although mayflies inhabit a variety of habitats in streams, differences in nematode presence could be expected as free living nematodes vary in streams according to substrate, water flow, and dissolved oxygen (Hodda, 2006). In contrast to temperate regions, there is limited information on nematode occurrence in mayflies from tropical regions. However, there are reports on other groups of aquatic insects. For example, a study from Venezuela reported large infection rates on stonefly nymphs (Plecoptera) in a stream (Gamboa et al., 2012).
In this study, we conducted a survey of mayflies in a forested stream in Puerto Rico to determine whether nematodes are present on Ephemeroptera. Our goals were to: (1) assess the presence, location within the body, and form of nematodes in mayflies; and (2) assess nematode frequency among taxa, stream habitat, and mayfly body size. To our knowledge, this is the first study assessing nematode presence in mayflies in Puerto Rico.
MATERIALS AND METHODS
The study was conducted at El Verde Field Station, within the Luquillo Experimental Forest (LEF), Puerto Rico. The LEF is located in the Northeast part of the island with elevations up to 1074 m and little rainfall seasonality, except for a drier period during the first part of the year (McDowell et al., 2012). At the field station, mean monthly precipitation varies from 200 to 300 mm and mean daily air temperature from 21.5 to 23.5 °C, with highest temperatures in June-July and lowest in January (McDowell et al., 2012). Mayflies were collected from Prieta stream.
Mayflies were collected using a D net (mesh size = 250 μm) by disturbing the substrate in three major stream habitats: riffles, pools, and boulders. In boulders, we gently scrubbed the rock surface by hand to avoid damaging specimens. Five replicates were collected, each consisting of three kick samples of three minutes. Nymphs were transported alive, placed in an aerated plastic aquarium at the station, and maintained alive until dissections and nematode observations were made.
Nematode presence was determined under a light microscope (either 4 x or 10 x). Mayflies were placed on a drop of water between a microscope slide and a glass cover to immobilize them, we used stacked glass covers on the edges (2 - 4) to avoid damaging the mayfly (Hominick and Welch, 1980). Mayflies were identified to species using the keys in Traver (1938) and our reference collection, and classified as infected or uninfected after examination. If nematodes were observed, we recorded the location of the nematode, its shape and movement, and whether it was encysted or not (Poinar and Thomas, 1984). Several nymphs were dissected for preservation of the nematodes. Dissections occurred in a 0.9 % NaCl solution to prevent nematodes from breaking due to osmotic changes (Poinar and Thomas, 1984) and to promote an easier transition from the host body to the slides. To facilitate the identification, nematodes were fixed on the slides with a flame, stained with McCormick Schilling red food color for a 12 hr period (Thies et al., 2002), and fixed with glycerol. The slides were sealed with clear nail polish (Poinar and Thomas, 1984; Kleynhans, 1999).
Nematode infection is reported as prevalence, or the proportion of infected mayflies. Chi- squared was used to test for significant statistical differences among families or species. Differences in nematode infection per habitat were tested with Kruskal-Wallis, as data was not normal. We used linear regressions between individual nymph body length (mm) and nematode presence by species. All statistical analysis was run in PAST, version 3.14 (Hammer et al., 2001).
RESULTS
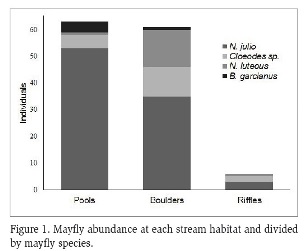
A total of 130 mayfly nymphs were collected from Prieta stream during June-July, 2016. Mayflies belonged to two families with two species each: Leptophlebiidae with Neohagenulus julio (Traver, 1938) and Neohagenulus luteous (Traver, 1938); and Baetidae with Baetis garcianus (Traver, 1938) and Cloeodes sp. (Traver, 1938). Species relative abundance at the stream was 70 % for N. julio, 12 % for N. luteous, 14 % for Cloeodes sp. and 4 % for B. garcianus. Pools and boulders were the habitats with the highest number of mayflies, followed by riffles (Figure 1). N. julio was the most abundant species in all habitats (Figure 1).

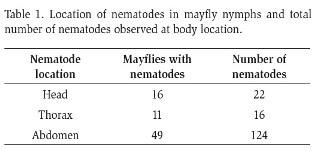
Nematodes were evident as clear, slim, elongated wormlike bodies moving within mayflies. They were mostly found moving freely in the abdomen and in some specimens in the head or thorax (Table 1). Mayfly antennae, caudal appendages, and legs were not infected. Nematodes were observed in different positions: straight, loop, and spiral. Encysted nematodes were rare (n = 11), while moving specimens were the norm (n = 119). Nematodes observed near or on top of the digestive tract were more passive and showed slow or no movement. Two mayflies were found with a bundle of ~12 spiraled nematodes in the abdominal cavity. Egg masses that resemble those of nematodes were observed in the abdomen and head of mayflies.
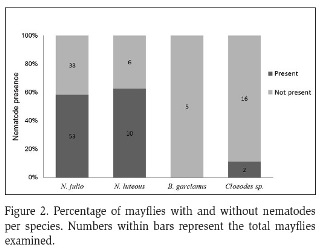
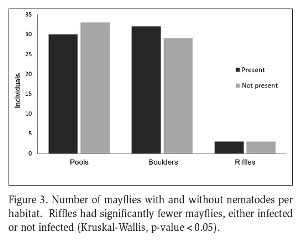
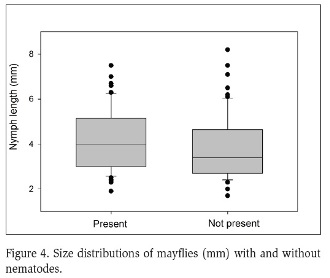
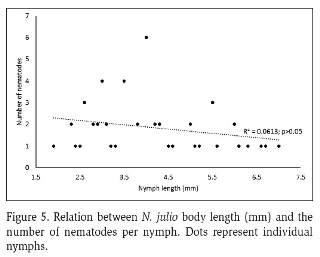
Average nematode prevalence was 50 %. Nematodes were not found in B. garcianus, had low presence in Cloeodes sp. (11 %), and were abundant in N. julio (58 %) and N. luteous (62 %) (Figure 2). Nematode presence was significantly different between Leptophlebiidae and Baetidae (Chi-squared test, p-value<0.05), but not significant between Neohagenulus species (Chi-squared test, p-value>0.97). Among habitats, boulders and pools were the habitats with the most infected mayflies, with few in riffles (Figure 3). Nematode presence was not related to mayfly nymph size, neither as a group (Figure 4) nor for N. julio, the most abundant species (Figure 5).
DISCUSSION
Nematodes were a common occurrence at the study stream and we suspect in other streams on the island as well. Although we did not identify them beyond phylum level (Nematoda), we can rule out two major groups based on morphology and effects: Gordiida (Nematomorpha) and Mermithidae (Nematoda). Gordian worms were disregarded based on differences in size, color, and life cycle. Gordian worms have long, slender, dark bodies (Poinar and Thomas, 1984), we only observed short worms with no color. In addition, Nematomorpha juveniles cross the gut wall and encyst (Nickle, 1972; Hominick and Welch, 1980; Looney et al., 2012) and we only observed few of these cases. Mermithids were discarded as an option, as they are whitish nematodes, often several times the length of their hosts when mature (Poinar, 1975; Poinar and Thomas, 1984) and are mostly found as one individual per host (Vance and Peckarsky, 1996), coiled and occupying most of the host haemocoel (Hominick and Welch, 1980). Also, a mermithid nematode would change the body wall color (towards transparency) of their host (Hominick and Welch, 1980), which we did not observed.
The location of nematodes in the mayfly body cavities and haemocoel, instead of being encysted, offer two possible interactions between mayflies and nematodes. First, the roundworm may be an internal phoretic nematode, potentially ingested as a low-movement, substrate dweller, juvenile, while the mayfly feed. Endo-phoresy between nematodes and aquatic insects has been documented in chironomid and mosquito fossils (Poinar, 2003). A second possible interaction is that the nematode is an obligate parasite that uses mayflies as intermediate host. This idea agrees with the observation of many small nematodes, similar to well known cases of intermediate hosts. For example, spirurid nematodes (Tetramermis fissispina) parasite ducks and amphibians and use mayflies as intermediate hosts (Garkavi, 1965, cited in Poinar, 2015, p. 292). Nematode eggs hatch in the mayfly, enter the body cavity, and develop until reaching an infective stage, normally a small worm that can be observed with simple microscopic examination (Poinar, 1975; 2015). Either case would represent a low impact on mayfly fitness.
Different proportions of infection among mayfly species could be the result of body shape, feeding strategy, and nymph activity pattern. This have been reported for mosquitoes, in which larval activity and physical characteristics influenced nematode infection (Petersen, 1975). Leptophlebiidae nymphs have flat and wide bodies that are in close contact with the substrate. They also consume a large amount of small particles (Cross et al., 2008). In contrast, Baetidae are cylindrically shaped and more hydrodynamic, and do not press the body against the substrate (Domínguez et al., 1995). Their feeding strategy involves the consumption mostly of diatoms, like typical scrapers (Ramírez and Gutiérrez-Fonseca, 2014). In addition, Leptophlebiidae are mostly crawlers, while Baetidae are swimmers. Body and behavioral characteristics may influence nematode access to their host and these characteristics potentially explain differences in infection among families and species.
Mayfly species composition was typical for streams at the LEF. N. julio and Cloeodes, the most abundant species in this study were also found abundant in an emergence study by Pescador et al. (1993). Differences among habitats in mayfly abundance represent habitat preference by different species and also food availability (e.g., more periphyton on boulders; Macías et al., 2014). Fewer mayflies and fewer infected individuals in riffles might be the result of substrate stability or sedimentation. Contrary to expectations, larger specimens did not have more nematodes, as could be expected due to the greater exposure time of mature nymphs. Overall, nematode infection was common and could represent an important impact on mayfly fitness and population dynamics that deserves further attention.
ACKNOWLEDGEMENTS
We thank Heidi Reyes, Director of the Biology Department, University of Puerto Rico at Ponce, for essential materials for this research. We also thank El Verde Field Station staff and Onanji Mijoso for help during sampling. Funding was obtained through the REU Site program at El Verde Field Station, University of Puerto Rico (NSF, DBI-1559679). The manuscript was improved with comments from the anonymous reviewers.
REFERENCES
Cross, W.F., Ramírez, A., Santana, A. and Silvestrini, L. 2008. Toward separating the relative importance of invertebrate consumption and bioturbation in Puerto Rican streams. Biotropica 40(4): 477–484.
Domínguez, E., Hubbard, M.D. and Peters, W.L. 1995. Insecta Ephemeroptera. In: Lopretto, E.C. and Tell, G., Editors. Ecosistemas de Aguas Continentales: Metodologías para su estudio. Ediciones Sur, La Plata.
Gamboa, M., Castillo, M.M. and Guerrero, R. 2012. Anacroneuria spp. (Insecta: Plecoptera: Perlidae) as paratenic hosts of Pheromermis sp. (Nematoda: Mermithidae) in Venezuela. Nematology 14(2): 185–190.
Giblin-Davis, R.M., Kanzaki, N. and Davies, K.A. 2013. Nematodes that Ride Insects: Unforeseen Consequences of Arriving Species. Florida Entomologist 96(3): 770–780.
Hammer, Ø., Harper, D.A.T. and Ryan, P.D. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4(1): 1–9.
Hodda, M. 2006. Nematodes in lotic systems. In: EyualemAbebe, E., Traunspurger, W. and Andrassy, I., Editors. Freshwater nematodes: ecology and taxonomy. CABI Publishing, Wallingford, United Kingdom and Cambridge, Massachusetts.
Hominick, W. and Welch, H. 1980. Mermithids (Nematoda) and Mayflies (Ephemeroptera). In: Flannagan, J. and Marshall, K., Editors. Advances in Ephemeroptera Biology. Springer, New York.
Kleynhans, K.P. 1999. Collecting and Preserving Nematodes: A Manual for Nematology. ARC-Plant Protection Research Institute, Pretoria, South Africa.
Looney, C., Hanelt, B. and Zack, R. 2012. New records of nematomorph parasites (Nematomorpha: Gordiida) of ground beetles (Coleoptera: Carabidae) and camel crickets (Orthoptera: Rhaphidophoridae) in Washington State. Journal of Parasitology 98(3): 554–559.
Macías, N.A., Colón-Gaud, C., Duggins, J.W. and Ramírez, A. 2014. Do omnivorous shrimp influence mayfly nymph life history traits in a tropical island stream? Revista de Biología Tropical 62(2): 41–51.
McDowell, W.H., Scatena, F.N., Waide, R.B., Brokaw, N., Camilo, G.R., Covich, A.P., Crowl, T.A., González, G., Greathouse, E.A., Klawinski, P., Lodge, D.J., Lugo, A.E., Pringle, C.M., Richardson, B.A., Richardson, M.J., Schaefer, D.A., Silver, W.L., Thompson, J., Vogt, D.J., Vogt, K.A., Willig, M.R., Woolbright, L.L., Zou, X. and Zimmerman, J. 2012. Geographic and Ecological Setting of the Luquillo Mountains. In: Brokaw, N., Crowl, T.A., Lugo, A.E., McDowell, W.H., Scatena, F.N., Waide, R.B. and Willig, M.R., Editors. A Caribbean Forest Tapestry, The multidimensional nature of disturbance and response. Oxford University Press, New York.
Nickle, W.R. 1972. A Contribution to our Knowledge of the Mermithidae (Nematoda). Journal of Nematology 4(2): 113–146.
Pescador, M.L., Masteller, E.C. and Buzby, K.M. 1993. Composition and Phenology of Ephemeroptera from a Tropical Rainforest Stream at El Verde, Puerto Rico. Journal of the Kansas Entomological Society 66(2): 151–159.
Petersen, J.J. 1975. Penetration and Development of the Mermithid Nematode Reesimermis nielseni in Eighteen Species of Mosquitoes. Journal of Nematology 7(3): 207–210.
Poinar, G.O. 1975. Entomogenous Nematodes: A Manual and Host List of Insect-Nematode Associations. Brill Archive, Leiden.
Poinar, G.O. 2003. Trends in the evolution of insect parasitism by nematodes as inferred from fossil evidence. Journal of nematology 35(2): 129–132.
Poinar, G.O. 2015. Phylum Nemata. In: Thorp, J.H. and Rogers, D.C., Editors. Thorp and Covich’s Freshwater Invertebrates Ecology and General Biology. Elsevier, Amsterdam.
Poinar, G.O. and Thomas, G. 1984. Laboratory Guide to Insect Pathogens and Parasites. Plenum Press, New York.
Ramírez, A. and Gutiérrez-Fonseca, P.E. 2014. Functional feeding groups of aquatic insect families in Latin America: a critical analysis and review of existing literature. Revista de Biología Tropical 62(Supplement 2): 155–167.
Sudhaus, W. 2008. Evolution of insect parasitism in Rhabditid and Diplogastrid nematodes. Advances in arachnology and developmental biology 12: 143–161.
Svensson, B.S. 1979. Pupation, emergence and fecundity of phoretic Epoicocladius ephemerae (Chironomidae). Holarctic Ecology 2(1): 41–50.
Thies, J.A., Merrill, S.B. and Corley, E.L. 2002. Red Food Coloring Stain: New, Safer Procedures for Staining Nematodes in Roots and Egg Masses on Root Surfaces. Journal of Nematology 34(2): 179–181.
Traver, J.R. 1938. Mayflies of Puerto Rico. Journal of Agriculture of the University of Puerto Rico 22(1): 5–42.
Vance, S. and Peckarsky, B. 1996. The infection of nymphal Baetis bicaudatus (Ephemeroptera) by the mermithid nematode Gasteromermis sp. Ecological Entomology 221: 377–381.
Vance, S. and Peckarsky, B. 1997. The effect of mermithid parasitism on predation of nymphal Baetis bicaudatus (Ephemeroptera) by invertebrates. Oceologia 110:147–152.
Williams, J.K., Townsend, C.F. and Poulin, R. 2001. Mermithid nematode infections and drift in the mayfly Deleatidium spp. (Ephemeroptera). Journal of Parasitology 87(5): 1225–1227.
Fecha de Recibido: 30/09/2016
Fecha de Aceptación: 22/11/2016
Para citar este artículo: Reyes-Torres, L.J., Meléndez-Torres, Y. and Ramírez, A. 2016. Occurrence of nematodes on Ephemeroptera nymphs in a tropical rainforest stream. Revista Intropica Vol. 11: 67 - 72