URBAN BLOW FLIES (DIPTERA: CALLIPHORIDAE) IN FOUR CITIES OF
THE COLOMBIAN CARIBBEAN COAST
Melissa Santodomingo-M., Adriana Santodomingo-Santodomingo y César Valverde-C.
Dirección de los autores:
Programa de Biología, Universidad del Magdalena, Santa Marta, Colombia. e-mail: melissa.santodomingo@hotmail.com (M.S.M.) adrianasantodomingo@gmail.com (A.S.S.), Grupo de Entomología (G.E.U.A.), Universidad de Antioquia, Calle 67 #53-108 Bloque 7-311. Medellin-Colombia. E-mail: cesarvalverdec@gmail.com (C.V.C.)
Fecha de Recepción: 05/04/2014
Fecha de Aceptación: 20/08/2014
Para citar este artículo: Santodomingo-M, M., A, Santodomingo-Santodomingo y C. Valverde-C. 2014. Urban blow flies (Diptera: Calliphoridae) in four cities of the Colombian Caribbean Coast. Revista Intropica 9: 84 - 91
ABSTRACT
The purpose of this study was to evaluate the species composition of the family Calliphoridae from samples collected in four different cities in the colombian Caribbean coast. Van Someren-Rydon traps were used baited with human faeces, rotten fish and fermented fruit were used. Six traps were placed in each city (two traps per type of bait), for a total of 24 traps. They were left for 72 hours in each site and samples were collected every 12 hours (day and night). 5654 individuals were identified, belonging to the subfamilies Chrysomyinae and Luciliinae. The identified species were Cochliomyia macellaria, Chrysomya albiceps, Chrysomya megacephala, Lucilia eximia, Lucilia sericata and Chloroprocta idioidea, expanding the range of distribution for the last two species. The best bait was the rotten fish and the best time to collect these species was during daylight.
KEY WORDS: preferred bait, Lucilia sericata, Chloroprocta idioidea, circadian cycle
CALIFÓRIDOS URBANOS (DIPTERA: CALLIPHORIDAE) EN CUATRO CIUDADES DE LA
COSTA CARIBE COLOMBIANA
RESUMEN
El propósito de este estudio fue evaluar la composición de las especies de la familia Calliphoridae recolectadas en cuatro diferentes ciudades de la costa Caribe colombiana. Se utilizaron trampas Van Someren-Rydon cebadas con heces humanas, pescado descompuesto y fruta fermentada. Seis trampas fueron colocadas en cada ciudad (dos trampas por cebo), para un total de 24 trampas. Estas se dejaron durante 72 horas en cada sitio y se recogieron muestras cada 12 horas (día y noche). Se identificaron 5654 individuos, pertenecientes a las subfamilias Chrysomyinae y Luciliinae. Las especies identificadas fueron Cochliomyia macellaria, Chrysomya albiceps, Chrysomya megacephala, Lucilia eximia, Lucilia sericata y Chloroprocta idioidea, ampliando el rango de distribución de las dos últimas especies. El cebo más efectivo fue el pescado descompuesto y el mejor momento para recolectar estas especies fue durante el día.
PALABRAS CLAVE: preferencia de cebo, Lucilia sericata, Chloroprocta idioidea, ciclo circadiano
INTRODUCTION
One of the main groups of insects with the greatest impact on human society is dipterans, which represent 12 % of known animal species (Pape and Evenhuis, 2013). The Calliphoridae family includes flies that are mostly of bright metallic colors (blue or green) and are generally decomposers, although there are also sarcosaprophagous, coprophagous (Amat et al., 2008), necrophagus, predators and parasitoid species (Amat, 2009); also the fact that they live in unhealthy urban settlements allows them to be mechanical propagators of human enteropathogens (Graczyk et al., 2005). The group plays an important role in the process of decomposition of organic matter, because of its high predominance and complex ecological communities (Hanski, 1987; Shewell, 1987).
There are few studies on the family Calliphoridae in the north area of South America, some are limited to type localities or to individuals collected by professionals from other countries (Amat, 2009). In Colombia there are 29 species and 12 genera grouped into 3 subfamilies: Calliphorinae, Chrysomyinae and Toxotarsinae (Amat et al., 2008).
In the past few years, some studies of forensic importance have been carried out on Calliphoridae in Colombia. Calliphoridae is the first group of insects that colonize corpses, enabling scientists to determine the post-mortem interval. However, there are no specific revisions about the species found in the Colombian Caribbean. There are reports of the species Lucilia eximia in the departments of Bolívar and Magdalena; Chrysomya megacephala in the departments of Sucre, Bolívar and Magdalena; Cochliomyia macellaria in all regions up to 2.500 meters high; Chloroprocta idioidea in the department of Sucre and Bolívar; Lucilia sericata in the departments of Sucre and La Guajira; Chrysomya albiceps in the departments of Sucre and Magdalena; and Roraimomusca roraima, Blepharignema splendens, Eumensembrinella quadrilineata and Hemilucilia sp. in the Sierra Nevada of Santa Marta (SNSM) (Pape et al., 2004; Amat and Wolff, 2007; Amat, 2009; Wolff et al., 2010a; Giraldo et al., 2011; Solano et al., 2013).
Due to the Calliphoridae's medical and sanitary importance as being transmitters of pathogens, their close location to urban settlements, and their usefulness as a tool in forensic studies, it is necessary to have baseline information about this family in every region of the country. The objective of this study was to evaluate the species composition of blowflies collected in four cities of the colombian Caribbean coast and to describe their preferences in bait and dial behavior in order to increase knowledge of the ecology of this family.
MATERIALS AND METHODS
Study Area
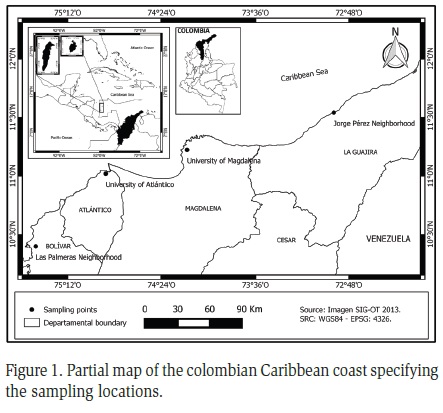
The samples were collected in the colombian Caribbean between July 24 and August 29, 2010. The study area corresponded to the "zonobioma subxerofitico tropical" which has a temperature between 25 and 38 °C and an annual rainfall of less than 2000 mm. This can be considered a transitional biome between the "zonobioma subxerofitico tropical" and the "zonobiomadesértico tropical" (Hernández and Sánchez, 1992). The region has an arid and semi-arid climate with low precipitation both in the summer and in the rainy season. "Unimodal-Biestacional" and "Bimodal-Tetraestacional" rainfall regime are common in this area (Rangel-Ch and Carvajal-Cogollo, 2012). The vegetation of this zonobiome has structural adaptations such as the presence of compound leaves, small leaflets, and thorns (IAVH, 1995). Canopy height ranges from 15 to 25 m and are presented as four vegetative strata including the herbaceous stratum (IAVH, 1998). Four coastal localities were selected (Figure 1):
The University of Magdalena (11°13'18.36"N, 74°11'10.89"W, 20 meters above sea level) in the District of Santa Marta, department of Magdalena. Sampling was conducted in the experimental farm of the university, within the dry forest plot (2 ha), a secondary forest in state of succession, bordered by grassland area of crops and fish ponds. This area is located 100 m from settlements (Strewe et al., 2009).
The Jorge Pérez neighborhood (11°32'25.02"N, 72°55'47.45"W, 10 meters above sea level) in Riohacha city, department of La Guajira. It is a peri-urban neighborhood, located in the city limits, bordering indigenous and displaced settlements. The main activity is fishing as it is located 500 m from the coastal area El Faro.
The University of Atlántico (11°01'07.58"N, 74°52'28.19"W, 48 meters above sea level) in the municipality of Puerto Colombia, Metropolitan Area of Barranquilla, department of Atlántico. Located approximately 3 km from the Caribbean sea, close to the lakes El Cisne and Caujaral. Vegetation of tropical dry forest is observed and in the driest areas, grasslands and cactus. It is situated in the greater urban and academicals zone of Barranquilla and the collect was done in the surrounding of gardens, cafeterias and classrooms.
The neighborhood Las Palmeras (10°23'51.04"N, 75°28'28.29"W, 6 meters above sea level) in the District of Cartagena, department of Bolivar. It is located in the commune 7, to the northwestern of the city and 7 km from the Caribbean Sea. It has a low socioeconomic status but all its basic needs are covered. The traps were placed in the backyard of a private residence surrounded by mango trees.
Sampling Method
Six Van Someren-Rydon (Vsr) traps were placed along a linear 300 m transect in the different sampling sites, with a distance between traps of 50 m. Three types of bait were used to attract the flies: 1). Mix of fermented pineapple with banana and beer (fruit), 2). Rotten fish (fish) and 3). Human faeces, obtaining a total of two samples of the same bait in each transect. Sampling was conducted for 72 hours in a continuous way, to get two populations (day and night) by collecting samples every 12 hours (06:00h and 18:00h).
Preservation and Identification
The material was separated, quantified and identified following the taxonomic keys provided by Whitworth (2006), Amat et al. (2008) and Vargas and Wood (2010) and were compared with the specimens identified in the collection of this family deposited in the Colección de Entomología de la Universidad de Antioquia (CEUA). Identification, mounting and preservation of insects were conducted in the installations of the CEUA, where the specimens were stored.
Data Analysis
For the analysis of bait preference a correspondence analysis (CA) was performed using the statistical environment R, version 2.15.3 and abundance of species data (Nenadic and Greenacre, 2007).
RESULTS
A total of 5654 individuals of Calliphoridae were identified, 2192 males and 3462 females, distributed in 4 genera and 6 species belonging to the subfamilies Luciliinae and Chrysomyinae. The most abundant species was C. megacephala (Fabricius, 1794) with a relative abundance (RA) of 82%, followed by C. idiodea (Robineau-Desvoidy, 1830), L. eximia (Wiedemann, 1819), C. albiceps (Wiedemann, 1819), C. macellaria (Fabricius, 1775) and finally L. sericata (Meigen, 1826). The distributional range of C. idioidea was extended to the departments of La Guajira, Magdalena and Atlántico, and of L. sericata to the department of Bolivar (Table 1).
The collected individuals showed mainly diurnal activity in general. Females were more abundant throughout the sampling in all locations (Table 1). Most individuals were collected in the Department of Magdalena and they showed strong preference for the fish bait. The same pattern was observed in all departments except La Guajira, where the fruit bait was more successful.
L. sericata was the species with the lowest abundance throughout the study (RA 0.30 %) (Table 1), with only 17 individuals captured, all females. It was distributed in the departments of La Guajira and Bolivar (Table 2), and was moderately attracted by the fruit bait with a relative frequency (RF) of 70.58 % (Figure 2). The preferred baits for C. idioidea were fruit and fish species and were collected in daylight hours, particularly in the department of Magdalena (Table 2). Its relative abundance was 5.69 % (Table 1).
The species C. megacephala had the highest abundance, with 82.11 % (Table 1). It was mainly found in the department of Magdalena, preferentially drawn from the human faeces (RF 39.06 %) and fish baits (RF 49.98%) (Figure 2). Its activity was greatly associated with daytime hours (Table 2). Alike, L. eximia showed greater preference for the human faeces bait with RF of 48.9 %, with fruit bait in second place (Figure 2) and most individuals of this species were captured in the department of Magdalena. They showed higher activity in the daytime, were generally females, (Table 2) and showed a RF of 5.19% (Table 1). On the other hand, C. albiceps was found with a RF of 3.89% (Table 1); unlike the two latter, this species was strongly attracted to the fish bait (RF 91.81 %) (Figure 2). It was found especially in the departments of Magdalena and Atlántico, with diurnal habits basically, and over 50% were females (Table 2). Ultimately, C. macellaria showed a RF of 2.79% (Table 1). Most individuals were attracted by the fish bait (RF 55.06 %), with human faeces bait in second place (RF 32.27 %) (Figure 2). The species showed higher abundance in the department of Magdalena and had generally diurnal habits (Table 2).
DISCUSSION
There were found 6 of the 29 species, and 4 of the 12 genus described for Colombia. On the other hand, almost all the species which are reported for the colombian Caribbean were captured, except for Roraimomusca roraima, Blepharignema splendens, Eumensembrinella quadrilineata and Hemilucilia sp., reported in the SNSM. This could be because all sampling locations were below 50 meters above sea level. The species recorded with higher abundance are species with strong preference for human settlements (Montoya et al., 2009).
Most individuals had a daytime activity although species such as C. megacephala have nocturnal oviposition (Singh and Bharti, 2001). This might be explained by the fact that the flies detect the bait by smell and observing at close range, although the latter may be limited at night by the lack of light, as with drosophilids the sleep cycle of calliphorids can be affected by artificial light (Amendt et al., 2008). This study was conducted in urban areas, so the amount of light may affect the hours of activity of the studied species. Therefore, more studies are needed to help determine circadian cycles of species of blowflies.
L. sericata was the only one of the six species that was not present in all the departments sampled, being found only in La Guajira and Bolivar. This is a new record of this species for the department of Bolivar, since it was previously reported only in the departments of Cundinamarca (Pape et al., 2004; Camacho, 2005; Yusseff, 2006; Echeverry et al., 2009; Salazar, 2010; Segura et al., 2010), Antioquia (Pape et al., 2004; Salazar, 2010; Ramirez et al., 2012), and Santander and Sucre (Pape et al., 2004), although Wolff (2010) described this species with wide distribution in Colombia. The other five species were found in all departments. Individuals of L. sericata showed preferences for traps baited with fruit (Figure 2); however, some studies reported it as a ghoul species (Echeverryet al., 2009; Pinilla et al., 2010; Grassberg and Reiter, 2011). Only 17 individuals of this species were collected in this study; therefore, this is not a representative sample to determine the preference of bait.
The second new record for the area was the species C. idioidea, which had been reported in the department of Antioquia (Amat 2009; Montoya et al., 2009; Salazar, 2010), in the colombian Amazon (Amat, 2009; 2010), in the departments of Caldas, Meta, Risaralda and Vichada (Amat, 2009) and reported by Wolff (2010) in warm regions and downs. The results of Amat (2009) and Montoya et al. (2009) are consistent with this study, describing the species with a high preference for the fish attractant.
C. megacephala was the most abundant introduced species. This result is similar to the ones reported by Buitrago and Bermudez (2010) in Panama, Mello et al. (2004) in Brazil and Pérez et al. (2005) and Yusseff (2006) in Colombia. Its abundance in urban areas is consistent with Mello et al. (2004), Montoya et al. (2009) and Rodrigues-Guimarães et al. (2008), who described it as a synanthropic species. In this study, most individuals were diurnal, as was found by Montoya et al. (2009).
L. eximia was captured mostly in the attractive human faeces (Figure 2), contrary to what is recorded in other studies in which the main attractant was carrion (Wolff et al., 2004; Gião and Godoy, 2006; Wolff et al., 2010b; Beltran and Villa, 2011), although Gião and Godoy (2006) also suggested preferences for excrement. This species was reported by Wolff et al. (2010b) and Beltran and Villa (2011) with synanthropic features.
C. albiceps is a species that was described as synanthropic by Mello et al. (2004) and Rodrigues–Guimarães et al. (2008). They also reported this species as the second most abundant in their studies, which agree the study by Ramos and Wolff (2011). Nevertheless, it was not very abundant in this work. In the collection of this species, females were present in greater quantity than males, as it is shown in the results of the studies of Rodrigues-Guimarães et al. (2008).
Montoya et al. (2009) described C. macellaria primarily as a diurnal species, which is consistent with this study. They also reported this species as one of the most abundant; however, in our study C. macellaria was one of the species with lower abundance, which can be explained by the presence of C. albiceps, an introduced species which in its larval stage feeds on others larvae of flies, principally C. macellaria, significantly reducing their populations (Rosa et al., 2006).
The blowflies in this study were found mostly in the traps baited with fish, in second place with human faeces and at last with fruit (Figure 2). This behavior is consistent to the fact that animal tissues have higher percentage of fat and protein, necessary for the development of the early larval stages, consequently the most captured individuals were females (Stevens, 2003; Montoya et al., 2009; Charabidze et al., 2011; Rabelo et al., 2011; Battán et al., 2012). Human faeces and fruit baits probably provide a lesser amount of these nutrients than necessary for the development of larvae.
CONCLUSIONS
In the sampled departments genera Cochliomyia, Chrysomya, Lucilia and Chloroprocta were reported. These genera belong to the Chrysomyinae and Luciliinae subfamilies. The most abundant species in this study was C. megacephala, corroborating the fact that this species has a high synanthropy, hence a medical and public health importance, useful in studies of forensic entomology in the examined localities. In addition the range of distribution of L. sericata was extended to the department of Bolivar and C. idioidea to the departments of La Guajira, Magdalena, Bolívar and Atlántico. It would necessary a longer lasting study to determine if external factors are affecting the abundance of species of the Calliphoridae family in these locations.
In the colombian Caribbean coast there are no exhaustive studies in relation to the taxonomy and ecology of the species of the family Calliphoridae. For this reason, this study constitutes a significant contribution to the knowledge of this family in the north of Colombia.
ACKNOWLEDGEMENTS
We thank the Program for Young Researchers of COLCIENCIAS for funding this project, the Colección de Entomología de la Universidad de Antioquia (CEUA), especially the professor PhD Marta Wolff for their support in the taxonomic determination, to the University of Magdalena for all their logistical support. To Juan David Sánchez-R, Erick Perdomo Balaguera, Duvan P. Peluffo and Carlos Villa De León for their invaluable assistance in the field and all those who helped in one or another way in the realization of this project.
BIBLIOGRAFÍA
Amat, E. and M. Wolff. 2007. New records of Blepharicnema splendens (Calliphoridae: Calliphorinae, Luciliini) from Colombia. Revista de la Sociedad Entomológica Argentina 66: 187-190.
Amat, E., M. Vélez and M. Wolff. 2008. Clave ilustrada para la identificación de los géneros y las especies de califóridos (Diptera: Calliphoridae) de Colombia. Caldasia 30: 231-244.
Amat, E. 2009. Contribución al conocimiento de las Chrysomyinae y Toxotarsinae (Diptera: Calliphoridae) de Colombia. Revista Mexicana de Biodiversidad 80: 693-708.
Amat, E. 2010. Notes on necrophagus flies (Diptera:Calyptrate) associated with fish carrion in Colombian Amazon. Acta Amazónica 40(2): 397-400.
Amendt, J., R. Zehner and F. Reckel. 2008. The nocturnal oviposition behaviour of blow flies (Diptera: Calliphoridae) in Central Europe and its forensic implications. Forensic Science International 175: 61-64.
Battán Horenstein, M., B. Rosso and M. García. 2012. Seasonal structure and dynamics of sarcosaprophagous fauna on pig carrion in a rural area of Cordoba (Argentina): their importance in Forensic Science. Forensic Science International 217: 146-156.
Beltrán, A. and F. Villa. 2011. Sucesión de insectos en cadáveres de ratas Wistar (Muridae: Rattus norvegicus) (Berkenhout, 1769) en bosque húmedo premontano (Ibaguée- Colombia). Revista Tumbaga 6: 93-106.
Buitrago, Y. and S. Bermúdez. 2010. Sinantropía de Calliphoridae (Insecta: Diptera) en ciudad de Panamá, Panamá. Colombia. Memorias XXXI Congreso Sociedad Colombiana de Entomología, p. 35
Camacho, G. 2005. Sucesión de la entomofauna cadavérica y ciclo vital de Calliphora vicina (Diptera: Calliphoridae) como primera especie colonizadora, utilizando cerdo blanco (Sus scrofa) en Bogotá. Revista Colombiana de Entomología 31: 189-197.
Charabidze, D., B. Bourel and D. Gosset. 2011. Larval-mass effect: Characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Science International: 211: 61-66.
Echeverry, L., A. Zapata, A. Segura and F. Bello. 2009. Estudio de cultivos celulares primarios derivados de Lucilia sericata (Diptera: Calliphoridae). Revista Ciencias de la Salud 7(3): 17-28.
Gião, J. and W. Godoy. 2006. Seasonal Population Dynamics in Lucilia eximia (Wiedemann) (Diptera: Calliphoridae). Neotropical Entomology 35(6): 753-756.
Giraldo, P., S. Uribe and A. López. 2011. Análisis de secuencias de ADN mitocondrial (Cytb y ND1) en Lucilia eximia (Diptera: Calliphoridae). Revista Colombiana de Entomología 37(2): 273-278.
Graczyk, T., R. Knight and L. Tamang. 2005. Mechanical transmission of human protozoan parasites by insects. Clinical. Microbiology Reviews. 18: 128-132.
Grassberger, M. and C. Reiter. 2011. Effect of the temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen- and isomorphendiagram. Forensic Science International 120: 32-36.
Hanski, I. 1987. Carrion fly community dynamics: patchiness, seasonality and coexistence. Ecological Entomology 12: 257-266 pp. In: Wolff, M. 2010. Los Calliphoridae (DIPTERA). Boletín del Museo Entomológico, Francisco L. Gallego 2(2): 5-10.
Hernández-Camacho, J. and H. Sánchez Páez. 1992. Biomas terrestres de Colombia. pp 153-173. In :Halffter, G. (Ed). La Diversidad biológica iberoamericana I. Acta Zoológica Mexicana, CYTED-D, México. 390 p.
Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt, IAVH. 1995. Exploración ecológica a los fragmentos de bosque seco en el valle del río Magdalena (norte del departamento del Tolima). Grupo de exploraciones y monitoreo ambiental (GEMA). Villa de Leiva. Manuscrito inédito.
Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt, IAVH. 1998. Bosque seco tropical. pp 56- 71. In: Chávez, M. and N. Arango (Eds). Informe nacional sobre el estado de la biodiversidad en Colombia. Tomo 1. Ministerio del Medio Ambiente. PNUMA, Bogotá.
Mello, R., R. Gredilha and E. Guimãrães. 2004. Dados preliminares sobre sinantropia de Califorídeos (diptera: calliphoridae) no Município de Paracambi-rj. Revista da Universidade Rural, Série Ciências da Vida, UFRRJ Seropédica-RJ 24(2): 97-101.
Montoya, A., J. Sánchez and M. Wolff. 2009. Sinantropía de Calliphoridae (Diptera) del Municipio La Pintada, Antioquia- Colombia. Revista Colombiana de Entomología 35(1): 73-82.
Nenadic, O. and M. Greenacre. 2007. Correspondence analysis in R, with two- and three-dimensional graphics: The ca package. Journal of Statistical Software, 20 (3), http://cran.r-project.org, http://www.jstatsoft.org/v20/i03/
Pape, T. and N. Evenhuis. 2013. Systema Dipterorum. Version 1.5. URL: http://www.diptera.org/. Consultado: 15 de febrero del 2014.
Pape, T., M. Wolff and E. Amat. 2004. Los Califoridos, Estridos, Rinofóridos y Sarcofágidos (Díptera: Calliphoridae, Oestridae, Rinophoridae, Sarcophagidae) de Colombia. Biota Colombiana 5: 201-208.
Pérez, S., P. Duque and M. Wolff. 2005. Successional behavior and occurrence matrix of carrion-associated arthropods in the urban area of Medellin, Colombia. Journal of Forensic Science 50 (2): 448-454.
Pinilla, T., Y. Acuña, D. Cortes, D. Díaz, A. Segura and F. Bello. 2010. Características del ciclo biológico de Lucilia sericata (MEIGEN, 1826) (DIPTERA: CALLIPHORIDAE) sobre dietas diferentes. Revista U.D.C.A Actualidad y Divulgación Científica 13(2): 153-161.
Rabelo, K., P. Thyssen, R. Salgado, M. Araújo and S. Vasconcelos. 2011. Bionomics of two forensically important blowfly species Chrysomya megacephala and Chrysomya putoria (Diptera: Calliphoridae) reared on four types of diet. Forensic Science International 210(1): 257-262.
Ramirez, M., E. Buenaventura, L. Gomez and E. Amat. 2012. Updated checklist and new records of Calyptratae carrion flies (Diptera, Schizophora) from Valle de Aburrápand other localities in Colombia. Entomotropica 27(1): 27-35.
Ramos, Y. and M. Wolff. 2011. Entomofauna cadavérica asociada a cerdos expuestos al sol y sombra, en el Piedemonte Amazónico Colombiano. Momentos de Ciencia 8(1): 45-54.
Rangel-Ch, J.O. and J.E. Carvajal-Cogollo. 2012. Clima de la región Caribe colombiana. pp. 67-129. In: Rangel, J.O. (Ed). Colombia Diversidad Biótica XII La región Caribe de Colombia. Universidad Nacional de Colombia, Bogotá.
Rodrigues-Guimãraes, R., Guimãraes R, H. Barros, R. Carvalho and G. Moya-Borja. 2008. Sinantropia da Fauna de Califorídeos (Diptera, Calliphoridae) na Baixada Fluminense, Rio de Janeiro, Brasil. Revista de Ciencia & Tecnología 8(1): 22-33.
Rosa, G., L. De-Carvalho, S. Dos-Reis and W. Godoy. 2006. The dynamics of intraguild predation in Chrysomya albiceps Wied. (Diptera: Calliphoridae): Interactions between instars and species under different abundances of food. Neotropical Entomology 35(6): 775-780.
Salazar, J. 2010. Reporte de los fondos del MEFLG, Moscas (Diptera: Calliphoridae) del MEFLG. Boletín del Museo Entomológico Francisco Luís Gallego 2(2): 11-21.
Segura, N., M. Bonilla, W. Usaquén and F. Bello. 2010. Entomofauna resource distribution associated with pig cadavers in Bogotá DC. Medical and Veterinary Entomology 25(1): 46-52.
Singh, D. and M. Bharti. 2001. Further observations on the nocturnal oviposition behaviour of blow flies (Diptera: Calliphoridae). Forensic Science International 120: 124-126.
Shewell, G. 1987. Calliphoridae. pp 1133-1145. In: McAlpine, J., B. Paterson, G. Shewell, H. Teskey, J. Vockeroth and D. Wood. (Ed). Manual of Nearctic Diptera, Vol. 2. Otawa, Agriculture Canada, Monograph 28, 675-1332p.
Solano, J., M. Wolff and R. Castro. 2013. Identificación molecular de califóridos (Diptera: Calliphoridae) de importancia forense en Colombia. Revista Colombiana de Entomología 39(2): 281-290.
Stevens, J. 2003. The evolution of myiasis in blowflies (Calliphoridae). International Journal for Parasitology 33: 1105-1113.
Strewe, R., C. Villa-De León, J. Alzate, J. Beltrán, J. Moya, C. Navarro and G. Utria. 2009. Las Aves del Campus de la Universidad del Magdalena, Santa Marta, Colombia. Revista Intropica 4: 79-91.
Vargas, J., and M. Wood. 2010. Calliphoridae (Blow Flies). En: Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E. and Zumbado, M. (eds), A Manual of Central American Diptera, Vol. 2. NRC Research Press, Ottawa, pp. 1313-1335.
Whitworth, T. 2006. Claves para géneros y especies de moscas califóridas (Diptera: Calliphoridae) de América al norte de México. Proceedings of the Entomological Society of Washington 108(3): 689-725.
Wolff, M., M. Pérez and N. Giraldo. 2004. Estudio de la entomofauna cadavérica encontrada en humanos alrededor de Medellín, Colombia y su aplicación en la determinación del Intervalo Postmortem. Colombia. Memorias XXXI Congreso Sociedad Colombiana de Entomología.
Wolff, M. 2010. Los Calliphoridae (DIPTERA). Boletín del Museo Entomológico, Francisco L. Gallego 2(2): 5-10.
Wolff, M., E. Perdomo, C. Valverde and G. Mejía. 2010a. Insectos de importancia forense en cerdo blanco, en un bosque seco tropical (Santa Marta, Colombia).RESUMEN. Memorias XXXVII Congreso Sociedad Colombiana de Entomología, 188p.
Wolff, M., C. Rivera, S. Herrera, J. Wolff and M. Escobar. 2010b. Lucilia eximia (Diptera: Calliphoridae), una nueva alternativa para la terapia larval y reporte de casos en Colombia. IATREIA Revista Médica Universidad de Antioquia 23(2): 107-116.
Yusseff, S. 2006. Entomología forense: los insectos en la escena del crimen. Revista Luna Azul Universidad de Caldas 23: 42-49.